J Korean Med Sci.
2012 Jul;27(7):729-735. 10.3346/jkms.2012.27.7.729.
Innovative In Vitro Chemo-Hormonal Drug Therapy for Refractory Thyroid Carcinomas
- Affiliations
-
- 1Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 2Isu Abxis Co. Ltd., Seoul, Korea.
- 3Department of Surgery, Yonsei University College of Medicine, Seoul, Korea. woungyounc@yuhs.ac
- KMID: 2157915
- DOI: http://doi.org/10.3346/jkms.2012.27.7.729
Abstract
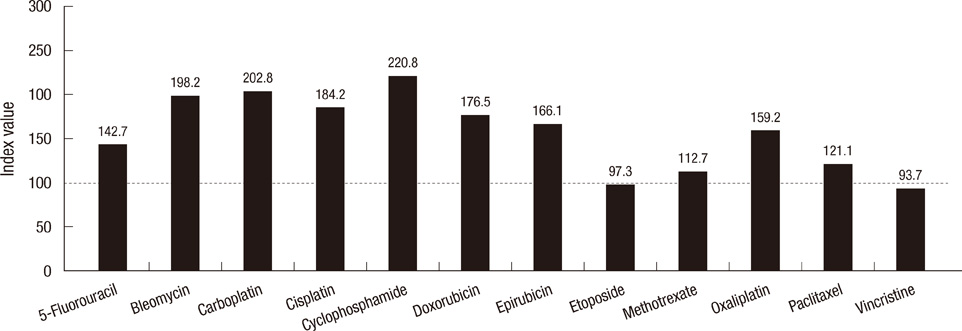
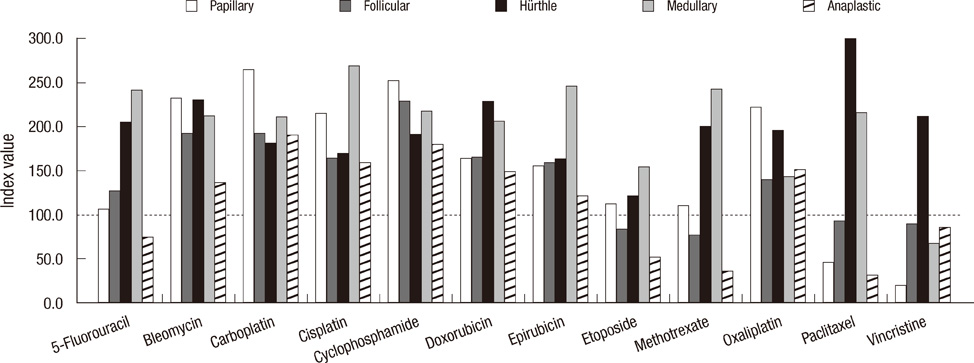
- More than 95% of the thyroid carcinomas are well differentiated types showing favorable prognosis. However, only a few therapeutic options are available to treat the patients with undifferentiated thyroid carcinomas, especially with refractory thyroid carcinomas that are not amenable to surgery or radioiodine ablation. We investigated the anticancer effects of 20 chemotherapy and hormonal therapy drugs on 8 thyroid carcinoma cell lines. In vitro chemosensitivity was tested using the adenosine-triphosphate-based chemotherapy response assay (ATP-CRA). The tumor inhibition rate (TIR; or cell death rate) or half maximal inhibitory concentration (IC50) was analyzed to interpret the results. Of the 12 chemotherapy drugs, etoposide (178.9 index value in follicular carcinoma cell line) and vincristine (211.7 in Hurthle cell carcinoma cell line) were the most active drugs showing the highest chemosensitivity, and of the 8 additional drugs, trichostatin A (0.03 microg/mL IC50 in follicular carcinoma cell line) showed favorable outcome having the anticancer effect. In our study, the result of etoposide and vincristine show evidence as active anticancer drugs in thyroid carcinoma cell lines and trichostatin A seems be the next promising drug. These drugs may become an innovative therapy for refractory thyroid carcinomas in near future.
Keyword
MeSH Terms
-
Adenosine Triphosphate/chemistry/pharmacology/therapeutic use
Antineoplastic Agents/chemistry/*pharmacology/therapeutic use
Apoptosis/drug effects
Cell Line, Tumor
Etoposide/chemistry/pharmacology/therapeutic use
Humans
Hydroxamic Acids/chemistry/pharmacology/therapeutic use
Thyroid Neoplasms/drug therapy
Vincristine/chemistry/pharmacology/therapeutic use
Antineoplastic Agents
Hydroxamic Acids
Etoposide
Adenosine Triphosphate
Vincristine
Figure
Reference
-
1. DeLellis RA. Pathology and genetics of thyroid carcinoma. J Surg Oncol. 2006. 94:662–669.2. Sherman SI. Thyroid carcinoma. Lancet. 2003. 361:501–511.3. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Sherman SI, Tuttle RM. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2006. 16:109–142.4. Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, Caillou B, Ricard M, Lumbroso JD, De Vathaire F, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006. 91:2892–2899.5. Heron DE, Karimpour S, Grigsby PW. Anaplastic thyroid carcinoma: comparison of conventional radiotherapy and hyperfractionation chemoradiotherapy in two groups. Am J Clin Oncol. 2002. 25:442–446.6. Are C, Shaha AR. Anaplastic thyroid carcinoma: biology, pathogenesis, prognostic factors, and treatment approaches. Ann Surg Oncol. 2006. 13:453–464.7. Stein R, Chen S, Reed L, Richel H, Goldenberg DM. Combining radioimmunotherapy and chemotherapy for treatment of medullary thyroid carcinoma: effectiveness of dacarbazine. Cancer. 2002. 94:51–61.8. Moley JF, Fialkowski EA. Evidence-based approach to the management of sporadic medullary thyroid carcinoma. World J Surg. 2007. 31:946–956.9. Pfister DG, Fagin JA. Refractory thyroid cancer: a paradigm shift in treatment is not far off. J Clin Oncol. 2008. 26:4701–4704.10. Pittas AG, Adler M, Fazzari M, Tickoo S, Rosai J, Larson SM, Robbins RJ. Bone metastases from thyroid carcinoma: clinical characteristics and prognostic variables in one hundred forty-six patients. Thyroid. 2000. 10:261–268.11. Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005. 12:245–262.12. Williams SD, Birch R, Einhorn LH. Phase II evaluation of doxorubicin plus cisplatin in advanced thyroid cancer: a Southeastern Cancer Study Group Trial. Cancer Treat Rep. 1986. 70:405–407.13. Pudney D, Lau H, Ruether JD, Falck V. Clinical experience of the multimodality management of anaplastic thyroid cancer and literature review. Thyroid. 2007. 17:1243–1250.14. Bajetta E, Rimassa L, Carnaghi C, Seregni E, Ferrari L, Di Bartolomeo M, Regalia E, Cassata A, Procopio G, Mariani L. 5-Fluorouracil, dacarbazine, and epirubicin in the treatment of patients with neuroendocrine tumors. Cancer. 1998. 83:372–378.15. Sherman SI. Early clinical studies of novel therapies for thyroid cancers. Endocrinol Metab Clin North Am. 2008. 37:511–524.16. Fagin JA. How thyroid tumors start and why it matters: kinase mutants as targets for solid cancer pharmacotherapy. J Endocrinol. 2004. 183:249–256.17. Laird AD, Cherrington JM. Small molecule tyrosine kinase inhibitors: clinical development of anticancer agents. Expert Opin Investig Drugs. 2003. 12:51–64.18. Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005. 438:967–974.19. Klein M, Vignaud JM, Hennequin V, Toussaint B, Bresler L, Plénat F, Leclère J, Duprez A, Weryha G. Increased expression of the vascular endothelial growth factor is a pejorative prognosis marker in papillary thyroid carcinoma. J Clin Endocrinol Metab. 2001. 86:656–658.20. Schweppe RE, Klopper JP, Korch C, Pugazhenthi U, Benezra M, Knauf JA, Fagin JA, Marlow LA, Copland JA, Smallridge RC, et al. Deoxyribonucleic acid profiling analysis of 40 human thyroid cancer cell lines reveals cross-contamination resulting in cell line redundancy and misidentification. J Clin Endocrinol Metab. 2008. 93:4331–4341.21. Moon YW, Choi SH, Kim YT, Sohn JH, Chang J, Kim SK, Park MS, Chung KY, Lee HJ, Kim JH. Adenosine triphosphate-based chemotherapy response assay (ATP-CRA)-guided platinum-based 2-drug chemotherapy for unresectable nonsmall-cell lung cancer. Cancer. 2007. 109:1829–1835.22. Kim HA, Yom CK, Moon BI, Choe KJ, Sung SH, Han WS, Choi HY, Kim HK, Park HK, Choi SH, et al. The use of an in vitro adenosine triphosphate-based chemotherapy response assay to predict chemotherapeutic response in breast cancer. Breast. 2008. 17:19–26.23. Han SS, Choi SH, Lee YK, Kim JW, Park NH, Song YS, Lee HP, Kang SB. Predictive value of individualized tumor response testing by ATP-based chemotherapy response assay in ovarian cancer. Cancer Invest. 2008. 26:426–430.24. Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973. 22:3099–3108.25. Huang YT, Hwang JJ, Lee PP, Ke FC, Huang JH, Huang CJ, Kandaswami C, Middleton E Jr, Lee MT. Effects of luteolin and quercetin, inhibitors of tyrosine kinase, on cell growth and metastasis-associated properties in A431 cells overexpressing epidermal growth factor receptor. Br J Pharmacol. 1999. 128:999–1010.26. Kang HJ, Youn YK, Hong MK, Kim LS. Antiproliferation and redifferentiation in thyroid cancer cell lines by polyphenol phytochemicals. J Korean Med Sci. 2011. 26:893–899.27. Sun CY, Hu Y, Guo T, Wang HF, Zhang XP, He WJ, Tan H. Resveratrol as a novel agent for treatment of multiple myeloma with matrix metalloproteinase inhibitory activity. Acta Pharmacol Sin. 2006. 27:1447–1452.28. Antonelli A, Ferrari SM, Fallahi P, Berti P, Materazzi G, Minuto M, Giannini R, Marchetti I, Barani L, Basolo F, et al. Thiazolidinediones and antiblastics in primary human anaplastic thyroid cancer cells. Clin Endocrinol (Oxf). 2009. 70:946–953.29. Yee KW, Schittenhelm M, O'Farrell AM, Town AR, McGreevey L, Bainbridge T, Cherrington JM, Heinrich MC. Synergistic effect of SU11248 with cytarabine or daunorubicin on FLT3 ITD-positive leukemic cells. Blood. 2004. 104:4202–4209.30. Petinari L, Kohn LK, de Carvalho JE, Genari SC. Cytotoxicity of tamoxifen in normal and tumoral cell lines and its ability to induce cellular transformation in vitro. Cell Biol Int. 2004. 28:531–539.31. Tuncel M, Aydin D, Yaman E, Tazebay UH, Güç D, Doğan AL, Taşbasan B, Uğur O. The comparative effects of gene modulators on thyroid-specific genes and radioiodine uptake. Cancer Biother Radiopharm. 2007. 22:443–449.32. Greenblatt DY, Cayo MA, Adler JT, Ning L, Haymart MR, Kunnimalaiyaan M, Chen H. Valproic acid activates Notch1 signaling and induces apoptosis in medullary thyroid cancer cells. Ann Surg. 2008. 247:1036–1040.33. Hoffmann S, Gläser S, Wunderlich A, Lingelbach S, Dietrich C, Burchert A, Müller H, Rothmund M, Zielke A. Targeting the EGF/VEGF-R system by tyrosine-kinase inhibitors: a novel antiproliferative/antiangiogenic strategy in thyroid cancer. Langenbecks Arch Surg. 2006. 391:589–596.34. Sherman SI. Targeted therapies for thyroid tumors. Mod Pathol. 2011. 24:S44–S52.35. Zarnegar R, Brunaud L, Kanauchi H, Wong M, Fung M, Ginzinger D, Duh QY, Clark OH. Increasing the effectiveness of radioactive iodine therapy in the treatment of thyroid cancer using Trichostatin A, a histone deacetylase inhibitor. Surgery. 2002. 132:984–990.36. Schlachterman A, Valle F, Wall KM, Azios NG, Castillo L, Morell L, Washington AV, Cubano LA, Dharmawardhane SF. Combined resveratrol, quercetin, and catechin treatment reduces breast tumor growth in a nude mouse model. Transl Oncol. 2008. 1:19–27.37. Kim DW, Jo YS, Jung HS, Chung HK, Song JH, Park KC, Park SH, Hwang JH, Rha SY, Kweon GR, et al. An orally administered multitarget tyrosine kinase inhibitor, SU11248, is a novel potent inhibitor of thyroid oncogenic RET/papillary thyroid cancer kinases. J Clin Endocrinol Metab. 2006. 91:4070–4076.38. Roman S, Mehta P, Sosa JA. Medullary thyroid cancer: early detection and novel treatments. Curr Opin Oncol. 2009. 21:5–10.39. Aljada A, O'Connor L, Fu YY, Mousa SA. PPAR gamma ligands, rosiglitazone and pioglitazone, inhibit bFGF- and VEGF-mediated angiogenesis. Angiogenesis. 2008. 11:361–367.40. Hoelting T, Siperstein AE, Duh QY, Clark OH. Tamoxifen inhibits growth, migration, and invasion of human follicular and papillary thyroid cancer cells in vitro and in vivo. J Clin Endocrinol Metab. 1995. 80:308–313.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- New Strategies for Combined Radioiodine Therapy in Refractory Thyroid Cancer
- Redifferentiation Therapy in Thyroid Cancer
- Successful Treatment of Cavernous Sinus Metastasis from Follicular Thyroid Carcinoma with Lenvatinib
- Management of Severe Fatigue Induced by Tyrosine Kinase Inhibitor in Radioiodine Refractory Thyroid Cancer
- Chemo-radiation Therapy for Locally Advanced Non-Small Cell Lung Cancer