J Korean Med Sci.
2005 Dec;20(6):932-937. 10.3346/jkms.2005.20.6.932.
Increased Anti-tumor Effect by a Combination of HSV Thymidine Kinase Suicide Gene Therapy and Interferon-gamma/GM-CSF Cytokine Gene Therapy in CT26 Tumor Model
- Affiliations
-
- 1Department of Internal Medicine, Korea Cancer Center Hospital, Seoul, Korea.
- 2Department of Internal Medicine, Chungbuk National University College of Medicine, Cheongju, Korea. stkim@chungbuk.ac.kr
- KMID: 2157739
- DOI: http://doi.org/10.3346/jkms.2005.20.6.932
Abstract
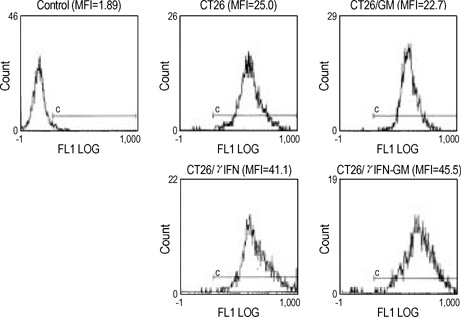
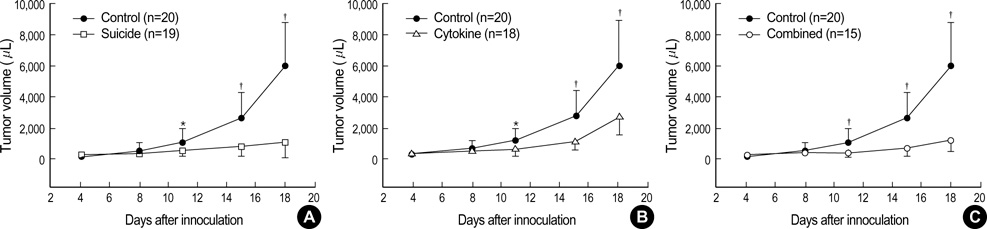
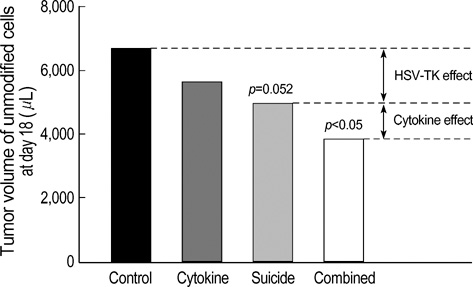
- The potential therapeutic benefit of introducing IFN-gamma and GM-CSF genes in combination with the HSVtk suicide gene into subcutaneously implanted CT26 tumor cells was compared with that from each treatment alone. Cells, unmodified or retrovirally transduced with HSVtk or IFN-gamma/GM-CSF genes, were inoculated subcutaneously into syngeneic BALB/c mice in various combinations. HSVtk gene, with intraperitoneal ganciclovir treatment, reduced tumor volume by 81% at locally inoculated tumor sites (p<0.01) and by 25% at distantly inoculated tumor sites (p=0.052). IFN-gamma/GM-CSF genes showed a 56% tumor volume reduction at local tumor sites (p<0.01) and 15% volume reduction at remote tumor sites, although this was not statistically significant. The combination of HSVtk (with GCV) and IFN-gamma/GM-CSF genes showed an 81% volume reduction at local tumor sites (p<0.01) and a 43% volume reduction at remote tumor sites (p<0.01). Thus, the combination of HSVtk and IFN-gamma/GM-CSF gene therapy produced greater therapeutic efficacy than either treatment alone.
Keyword
MeSH Terms
-
Animals
Cell Line
Cell Line, Tumor
Gene Therapy/*methods
Genes, Transgenic, Suicide
Granulocyte Macrophage Colony-Stimulating Factors, Recombinant/biosynthesis/*genetics/therapeutic use
H-2 Antigens/metabolism
Interferon-gamma, Recombinant/biosynthesis/*genetics/therapeutic use
Male
Mice
Mice, Inbred BALB C
Neoplasms, Experimental/*therapy
Research Support, Non-U.S. Gov't
Simplexvirus/enzymology/genetics
Thymidine Kinase/*genetics/therapeutic use
Transduction, Genetic
Figure
Reference
-
1. Dilber MS, Abedi MR, Bjorkstrand B, Christensson B, Gohrton G, Xanthogoulous KG, Smith CI. Suicide gene therapy for plasma cell tumors. Blood. 1996. 88:2192–2200.
Article2. Kim YG, Kim ST, Yoon HD. Enhanced in vitro and in vivo bystander effect by double transfer of herpes simplex virus thymidine kinase gene. J Korean Neurosurg Soc. 1999. 28:1407–1417.3. Kianmanesh AR, Perrin H, Panis Y, Fabre M, Nagy HJ, Houssin D, Klatzmann D. A distant bystander effect of suicide gene therapy; Regression of nontransduced tumors together with a distant transduced tumor. Human Gene Ther. 1997. 8:1807–1814.
Article4. Agard C, Ligeza C, Dupas B, Izembart A, Kouri CE, Moullier P, Ferry N. Immune-dependent distant bystander effect after adenovirus-mediated suicide gene transfer in a rat model of liver colorectal metastasis. Cancer Gene Ther. 2001. 8:128–136.
Article5. Fearson E, Hunt B, Itaya T, Frost P. Interleukin-2 production by tumor cells bypasses T helper function in the generation of an anti-tumor response. Cell. 1990. 60:397–403.
Article6. Patel PM, Fleming CL, Russel SJ, McKat IA, MacLennan KA, Box GM, Eccles SA, Collins MK. Comparison of the potential therapeutic effect of interleukin 2 or interleukin 4 secretion by a single tumor. Brit J Cancer. 1993. 68:295–305.7. Golumbek PT, Lazenby AJ, Levitsky HI, Jaffee LM, Karasuyama H, Baker M, Pardoll DM. Treatment of established renal cancer by tumor cells engineered to secrete IL-4. Science. 1991. 254:713–716.8. Esumi N, Hunt B, Itaya T, Frost P. Reduced tumorigenicity of murine tumor cells secreting IFN-γ is due to nonspecific host responses and is unrelated to class I major histocompatibility complex expression. Cancer Res. 1991. 51:1185–1189.9. Armstrong CA, Botella R, Galloway TH, Murray N, Kramp JM, Song IS, Ansel JC. Antitumour effects of granulocyte-macrophage colony-stimulating factor production by melanoma cells. Cancer Res. 1996. 56:2191–2198.10. Mesnil M, Piccoli C, Tiraby G, Willecke K, Yamasaki H. Bystander killing of cancer cells by herpes simplex virus thymidine kinase gene is mediated by connexins. Proc Natl Acad Sci USA. 1996. 93:1831–1835.
Article11. Burrows FJ, Gore M, Smiley WR, Kanemitsu MY, Jolly DJ, Read SB, Nicholas T, Kruse CA. Purified herpes simplex virus thymidine kinase retroviral particles; III. Characterization of bystander killing mechanisms in transfected tumor cells. Cancer Gene Ther. 2002. 9:87–95.12. Beck C, Cayeux S, Lupton SD, Dorken B, Blankenstein T. The thymidine kinase/ganciclovir-mediated suicide effect is variable in different tumor cells. Hum Gene Ther. 1995. 6:1525–1530.
Article13. Freeman SM, Whartenby KA, Freeman JL, Abboud CN, Marrogi AJ. In situ use of suicide genes for cancer therapy. Semin Oncol. 1996. 23:31–45.14. Caruso M, Paris Y, Gagandeep S, Houssin P, Saltzmann JL, Klatzmann D. Regression of established macroscopic liver metastases after in situ transduction of a suicide gene. Proc Natl Acad Sci USA. 1993. 90:7024–7028.
Article15. Weber JS, Rosenberg SA. Modulation of murine tumor major histocompatibility antigens by cytokines in vitro and in vivo. Cancer Res. 1988. 48:5818–5824.16. Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligar RC. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony stimulating factor stimulates potent, specific, and long lasting anti-tumor immunity. Proc Natl Acad Sci USA. 1993. 90:3539–3543.17. Kim ST, Lee KH, Oh TG, Kim YG. Effect of combined gene therapy using tricistronic retroviral vector containing gamma-interferon and granulocyte-macrophage colony-stimulating factor into CT26 cells. Korean J BRM. 1999. 9:41–49.18. Lee KH, Piao H, Son BR, Heo DS, Kim NK, Kim ST. Herpes simplex virus thymidine kinase and granulocyte macrophage colony-stimulating factor combination gene therapy in a murine CT26 cell colon cancer model. Cancer Gene Ther. 2004. 11:570–576.
Article19. Kruse CA, Roper MD, Kleinschmidt-DeMasters BK, Banuelos SJ, Smiley WR, Robbins JM, Burrows FJ. Purified herpes simplex thymidine kinase retrovector particles. I. In vitro characterization, in situ transduction efficiency, and histopathological analyses of gene therapy-treated brain tumors. Cancer Gene Ther. 1997. 4:118–128.20. Rockwell SC, Kallimann RF, Fajardo LF. Characteristics of a serially transplanted mouse mammary tumor and its tissue-culture-adapted derivative. J Natl Cancer Inst. 1972. 49:735–749.21. Ezzedine ZD, Martuza RL, Plaitika D. Selective killing of glioma cells in culture and in vivo by retrovirus transfer of the herpes simplex virus thymidine kinase gene. New Biol. 1991. 3:608–614.22. Culver KW, Ram Z, Wallbridge S, Ishii H, Oldfield EH, Blease RM. In vivo gene transfer with retroviral vector-producer cells for treatment of experimental brain tumors. Science. 1992. 256:1550–1552.
Article23. Pardoll DM. Paracrine cytokine adjuvants in cancer immunotherapy. Annu Rev Immunol. 1995. 13:399–415.
Article24. Chen SH, Kosai K, Xu B, Pham-Nguyen K, Contant C, Finegold MJ, Woo SL. Combination suicide and cytokine gene therapy for hepatic metastases of colon carcinoma: sustained antitumor immunity prolongs animal survival. Cancer Res. 1996. 56:3758–3762.25. Bonnekoh B, Greenhalgh DA, Chen SH, Block A, Rich SS, Kreig T, Woo SL, Roop DR. Ex vivo and in vivo adenovirus-mediated gene therapy strategies induce a systemic anti-tumor immune defence in the B16 melanoma model. J Invest Dermatol. 1998. 110:867–871.
Article26. Majumdar AS, Zolotorev A, Sammuel S, Tran K, Vertin B, Hall-Meier M, Antoni BA, Adeline E, Philip M, Philip R. Efficacy of herpes simplex virus thymidine kinase in combination with cytokine gene therapy in an experimental metastatic breast cancer model. Cancer Gene Ther. 2000. 7:1086–1099.
Article27. Toda M, Martuza RL, Rabkin SD. Combination suicide/cytokine gene therapy as adjuvants to a defective herpes simplex virus-based cancer vaccine. Gene Ther. 2001. 8:332–339.
Article28. North RJ. Down-regulation of the antitumor immune response. Adv Cancer Res. 1985. 45:1–43.
Article29. Maraskovsky E, Chen WF, Shortman K. IL-2 and IFN-gamma are two necessary lymphokines in the development of cytolytic T cells. J Immunol. 1989. 143:1210–1214.30. Saito S, Bannerji R, Gansbacher B, Rosenthal FM, Romanenko P, Heston WD, Fair WR, Gilboa E. Immunotherapy of bladder cancer with cytokine gene-modified tumor vaccines. Cancer Res. 1994. 54:3516–3520.31. Blaese RM, Ishii-Morita H, Mullen C, Ramsey J, Ram Z, Oldfield E, Culver K. In situ delivery of suicide genes for cancer treatment. Eur J Cancer. 1994. 30A:1190–1193.
Article32. Barba D, Hardin J, Sadelain M, Gage FH. Development of anti-tumor immunity following thymidine kinase-mediated killing of experimental brain tumors. Proc Natl Acad Sci USA. 1994. 91:4348–4352.
Article33. Gansbacher B, Bannerji R, Daniels B, Zier K, Cronin K, Gilboa E. Retroviral vector-mediated gamma-interferon gene transfer into tumor cells generates potent and long lasting anti-tumor immunity. Cancer Res. 1990. 50:7820–7825.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Application of Apoptogenic Pretreatment to Enhance Anti-tumor Immunity of Granulocyte-Macrophage Colony Stimulating Factor (GM-CSF)-secreting CT26 Tumor Cells
- Combination Gene Therapy of Herpes Simplex Virus Thymidine Kinase and Cytokines in Lung Cancer
- Increased Thymidine Kinase Activity and in vitro Bystander Effect by Double Transfer of HSVtk Gene into T98G Tumor Cells
- Adenovirus-mediated Suicide Gene Therapy for Experimental Prostate Cancers with in Vivo Tumor Transduction Using the Herpes Simplex Virus Thymidine Kinase Gene/Acyclovir System
- Antitunor effect of carcinoma cells transduced with herpes simplex virus - thymidine kinase by gancyclovir and radiation