Clin Exp Vaccine Res.
2016 Jan;5(1):50-59. 10.7774/cevr.2016.5.1.50.
A potent multivalent vaccine for modulation of immune system in atherosclerosis: an in silico approach
- Affiliations
-
- 1Cellular and Molecular Biology Research Center, Student Research Committee, School of Medicine, Babol University of Medical Sciences, Babol, Iran.
- 2Applied Microbiology Research Center, Baqiyatallah University of Medical Sciences, Tehran, Iran. Jafar.amani@gmail.com
- KMID: 2152697
- DOI: http://doi.org/10.7774/cevr.2016.5.1.50
Abstract
- PURPOSE
Atherosclerosis is classically defined as an immune-mediated disease characterized by accumulation of low-density lipoprotein cholesterol over intima in medium sized and large arteries. Recent studies have demonstrated that both innate and adaptive immune responses are involved in atherosclerosis. In addition, experimental and human models have recognized many autoantigens in pathophysiology of this disease. Oxidized low-density lipoproteins, beta2 glycoprotein I (beta-2-GPI), and heat shock protein 60 (HSP60) are the best studied of them which can represent promising approach to design worthwhile vaccines for modulation of atherosclerosis.
MATERIALS AND METHODS
In silico approaches are the best tools for design and evaluation of the vaccines before initiating the experimental study. In this study, we identified immunogenic epitopes of HSP60, ApoB-100, and beta-2-GPI as major antigens to construct a chimeric protein through bioinformatics tools. Additionally, we have evaluated physico-chemical properties, structures, stability, MHC binding properties, humoral and cellular immune responses, and allergenicity of this chimeric protein by means of bioinformatics tools and servers.
RESULTS
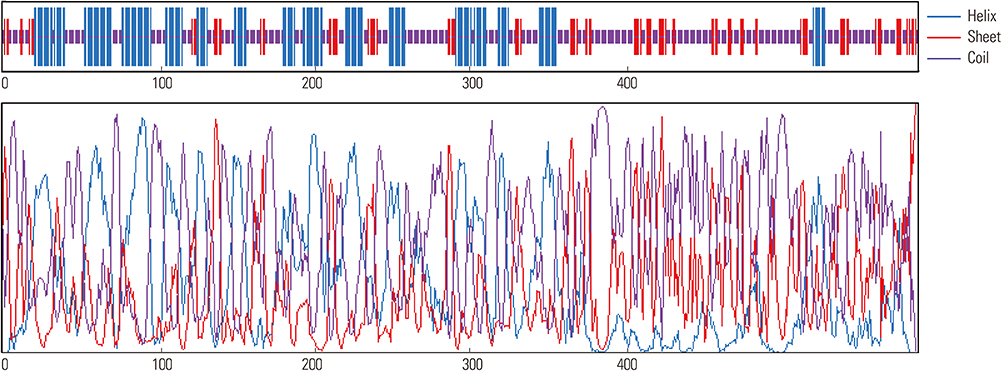
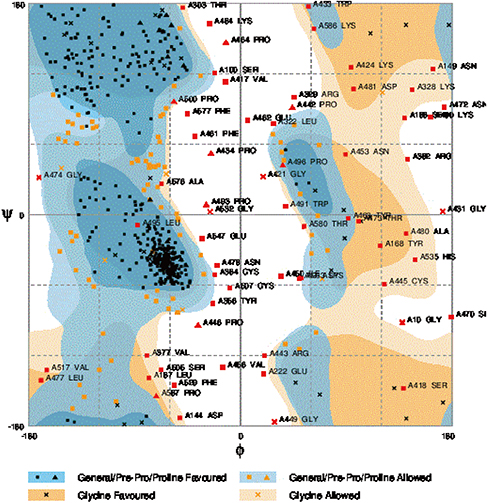
Validation results indicated that 89.1% residues locate in favorite or additional allowed region of Ramachandran plot. Also, based on Ramachandran plot analysis this protein could be classified as a stable fusion protein. In addition, the epitopes in the chimeric protein had strong potential to induce both the B-cell and T-cell mediated immune responses.
CONCLUSION
Our results supported that this chimeric vaccine could be effectively utilized as a multivalent vaccine for prevention and modulation of atherosclerosis.
MeSH Terms
-
Apolipoprotein B-100
Arteries
Atherosclerosis*
Autoantigens
B-Lymphocytes
beta 2-Glycoprotein I
Chaperonin 60
Cholesterol
Computational Biology
Computer Simulation*
Epitopes
Humans
Immune System*
Immunity, Cellular
Lipoproteins
Lipoproteins, LDL
T-Lymphocytes
Vaccines
Apolipoprotein B-100
Autoantigens
Chaperonin 60
Cholesterol
Epitopes
Lipoproteins
Lipoproteins, LDL
Vaccines
beta 2-Glycoprotein I
Figure
Reference
-
1. Tuttolomondo A, Di Raimondo D, Pecoraro R, Arnao V, Pinto A, Licata G. Atherosclerosis as an inflammatory disease. Curr Pharm Des. 2012; 18:4266–4288.
Article2. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005; 352:1685–1695.
Article3. Mizuno Y, Jacob RF, Mason RP. Inflammation and the development of atherosclerosis. J Atheroscler Thromb. 2011; 18:351–358.
Article4. Wick G, Jakic B, Buszko M, Wick MC, Grundtman C. The role of heat shock proteins in atherosclerosis. Nat Rev Cardiol. 2014; 11:516–529.
Article5. Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006; 6:508–519.
Article6. Businaro R, Tagliani A, Buttari B, et al. Cellular and molecular players in the atherosclerotic plaque progression. Ann N Y Acad Sci. 2012; 1262:134–141.
Article7. Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007; 116:1832–1844.
Article8. Nilsson J, Wigren M, Shah PK. Vaccines against atherosclerosis. Expert Rev Vaccines. 2013; 12:311–321.
Article9. Pichardo-Almarza C, Metcalf L, Finkelstein A, Diaz-Zuccarini V. Using a systems pharmacology approach to study the effect of statins on the early stage of atherosclerosis in humans. CPT Pharmacometrics Syst Pharmacol. 2015; 4:e00007.
Article10. Chyu KY, Shah PK. Advances in immune-modulating therapies to treat atherosclerotic cardiovascular diseases. Ther Adv Vaccines. 2014; 2:56–66.
Article11. Gero S, Gergely J, Jakab L, et al. Inhibition of cholesterol atherosclerosis by immunisation with beta-lipoprotein. Lancet. 1959; 2:6–7.
Article12. Chyu KY, Shah PK. Can we vaccinate against atherosclerosis? J Cardiovasc Pharmacol Ther. 2014; 19:77–82.
Article13. Chyu KY, Zhao X, Reyes OS, et al. Immunization using an Apo B-100 related epitope reduces atherosclerosis and plaque inflammation in hypercholesterolemic apo E (-/-) mice. Biochem Biophys Res Commun. 2005; 338:1982–1989.
Article14. Fredrikson GN, Soderberg I, Lindholm M, et al. Inhibition of atherosclerosis in apoE-null mice by immunization with apoB-100 peptide sequences. Arterioscler Thromb Vasc Biol. 2003; 23:879–884.
Article15. Nilsson J, Fredrikson GN, Bjorkbacka H, Chyu KY, Shah PK. Vaccines modulating lipoprotein autoimmunity as a possible future therapy for cardiovascular disease. J Intern Med. 2009; 266:221–231.
Article16. Kilic A, Mandal K. Heat shock proteins: pathogenic role in atherosclerosis and potential therapeutic implications. Autoimmune Dis. 2012; 2012:502813.
Article17. Long J, Lin J, Yang X, et al. Nasal immunization with different forms of heat shock protein-65 reduced high-cholesterol-diet-driven rabbit atherosclerosis. Int Immunopharmacol. 2012; 13:82–87.
Article18. Klingenberg R, Ketelhuth DF, Strodthoff D, Gregori S, Hansson GK. Subcutaneous immunization with heat shock protein-65 reduces atherosclerosis in Apoe(-)/(-) mice. Immunobiology. 2012; 217:540–547.
Article19. Lu X, Chen D, Endresz V, et al. Immunization with a combination of ApoB and HSP60 epitopes significantly reduces early atherosclerotic lesion in Apobtm2SgyLdlrtm1Her/J mice. Atherosclerosis. 2010; 212:472–480.
Article20. Serrano A, Garcia F, Serrano M, et al. IgA antibodies against beta2 glycoprotein I in hemodialysis patients are an independent risk factor for mortality. Kidney Int. 2012; 81:1239–1244.
Article21. De Groot PG, Meijers JC, Urbanus RT. Recent developments in our understanding of the antiphospholipid syndrome. Int J Lab Hematol. 2012; 34:223–231.
Article22. Banzato A, Pengo V. Clinical relevance of beta(2)-glycoprotein-I plasma levels in antiphospholipid syndrome (APS). Curr Rheumatol Rep. 2014; 16:424.23. Grote A, Hiller K, Scheer M, et al. JCat: a novel tool to adapt codon usage of a target gene to its potential expression host. Nucleic Acids Res. 2005; 33:W526–W531.
Article24. Doytchinova IA, Flower DR. VaxiJen: a server for prediction of protective antigens, tumour antigens and subunit vaccines. BMC Bioinformatics. 2007; 8:4.
Article25. Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003; 31:3406–3415.
Article26. Wilkins MR, Gasteiger E, Bairoch A, et al. Protein identification and analysis tools in the ExPASy server. Methods Mol Biol. 1999; 112:531–552.
Article27. Sen TZ, Jernigan RL, Garnier J, Kloczkowski A. GOR V server for protein secondary structure prediction. Bioinformatics. 2005; 21:2787–2788.
Article28. Zhang Y. I-TASSER server for protein 3D structure prediction. BMC Bioinformatics. 2008; 9:40.
Article29. El-Manzalawy Y, Dobbs D, Honavar V. Predicting flexible length linear B-cell epitopes. Comput Syst Bioinformatics Conf. 2008; 7:121–132.
Article30. Kringelum JV, Lundegaard C, Lund O, Nielsen M. Reliable B cell epitope predictions: impacts of method development and improved benchmarking. PLoS Comput Biol. 2012; 8:e1002829.
Article31. Bhasin M, Raghava GP. Prediction of CTL epitopes using QM, SVM and ANN techniques. Vaccine. 2004; 22:3195–3204.
Article32. Singh H, Raghava GP. ProPred1: prediction of promiscuous MHC class-I binding sites. Bioinformatics. 2003; 19:1009–1014.
Article33. Reche PA, Glutting JP, Reinherz EL. Prediction of MHC class I binding peptides using profile motifs. Hum Immunol. 2002; 63:701–709.
Article34. Saha S, Raghava GP. AlgPred: prediction of allergenic proteins and mapping of IgE epitopes. Nucleic Acids Res. 2006; 34:W202–W209.
Article35. Rammensee H, Bachmann J, Emmerich NP, Bachor OA, Stevanovic S. SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics. 1999; 50:213–219.
Article36. Brusic V, Rudy G, Harrison LC. MHCPEP, a database of MHC-binding peptides: update 1997. Nucleic Acids Res. 1998; 26:368–371.
Article37. de Jager SC, Kuiper J. Vaccination strategies in atherosclerosis. Thromb Haemost. 2011; 106:796–803.
Article38. Kimura T, Tse K, Sette A, Ley K. Vaccination to modulate atherosclerosis. Autoimmunity. 2015; 48:152–160.
Article39. de Groot PG, Meijers JC. beta(2) -Glycoprotein I: evolution, structure and function. J Thromb Haemost. 2011; 9:1275–1284.40. Willis R, Pierangeli SS. Anti-beta2-glycoprotein I antibodies. Ann N Y Acad Sci. 2013; 1285:44–58.41. Gaspar P, Moura G, Santos MA, Oliveira JL. mRNA secondary structure optimization using a correlated stem-loop prediction. Nucleic Acids Res. 2013; 41:e73.
Article42. Jahangiri A, Rasooli I, Reza Rahbar M, Khalili S, Amani J, Ahmadi Zanoos K. Precise detection of L. monocytogenes hitting its highly conserved region possessing several specific antibody binding sites. J Theor Biol. 2012; 305:15–23.
Article43. Rahbar MR, Rasooli I, Gargari SL, et al. A potential in silico antibody-antigen based diagnostic test for precise identification of Acinetobacter baumannii. J Theor Biol. 2012; 294:29–39.
Article44. Delfani S, Imani Fooladi AA, Mobarez AM, Emaneini M, Amani J, Sedighian H. In silico analysis for identifying potential vaccine candidates against Staphylococcus aureus. Clin Exp Vaccine Res. 2015; 4:99–106.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- In silico analysis of an envelope domain III-based multivalent fusion protein as a potential dengue vaccine candidate
- Protective efficacy of a novel multivalent vaccine in the prevention of diarrhea induced by enterotoxigenic Escherichia coli in a murine model
- Cancer Vaccines
- Microbiota Influences Vaccine and Mucosal Adjuvant Efficacy
- Vaccine adjuvant materials for cancer immunotherapy and control of infectious disease