Immune Netw.
2015 Oct;15(5):260-267. 10.4110/in.2015.15.5.260.
Engagement of CD99 Reduces AP-1 Activity by Inducing BATF in the Human Multiple Myeloma Cell Line RPMI8226
- Affiliations
-
- 1Cell Dysfunction Research Center, University of Ulsan College of Medicine, Seoul 05505, Korea. csikpark@amc.seoul.kr
- 2Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul 05505, Korea.
- 3SIS Immunology Research Center, Sookmyung Women's University, Seoul 04310, Korea.
- 4Institute for Life Sciences, Asan Medical Center, University of Ulsan College of Medicine, Seoul 05505, Korea.
- 5Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul 05505, Korea.
- 6Department of Otolaryngology, Asan Medical Center, University of Ulsan College of Medicine, Seoul 05505, Korea.
- KMID: 2150855
- DOI: http://doi.org/10.4110/in.2015.15.5.260
Abstract
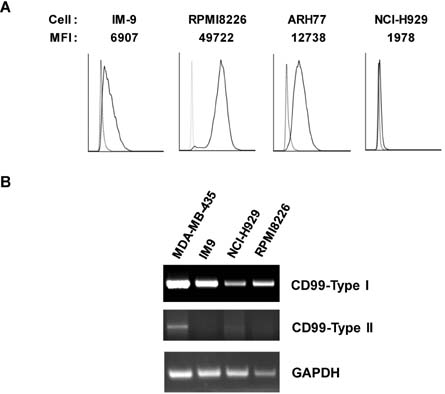
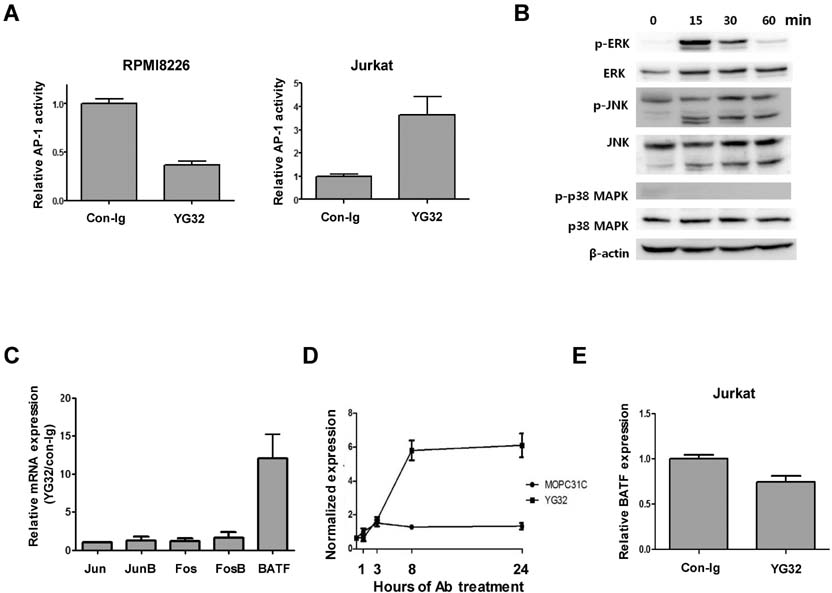
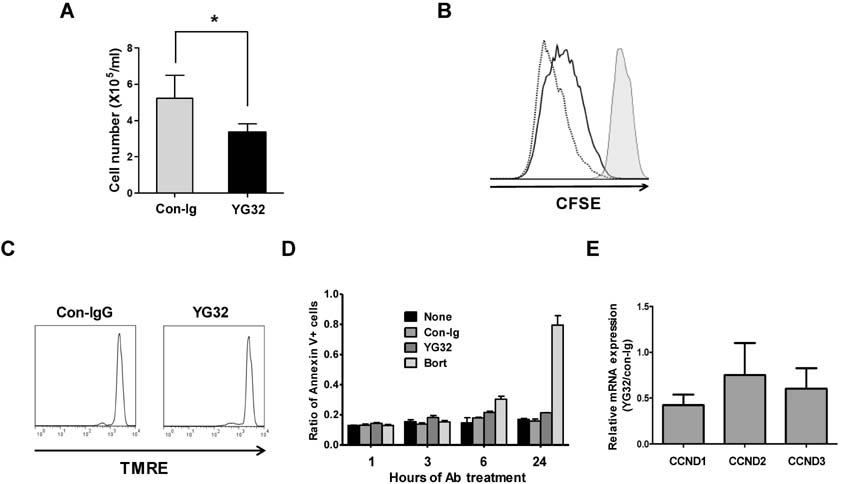
- CD99 signaling is crucial to a diverse range of biological functions including survival and proliferation. CD99 engagement is reported to augment activator protein-1 (AP-1) activity through mitogen-activated protein (MAP) kinase pathways in a T-lymphoblastic lymphoma cell line Jurkat and in breast cancer cell lines. In this study, we report that CD99 differentially regulated AP-1 activity in the human myeloma cell line RPMI8226. CD99 was highly expressed and the CD99 engagement led to activation of the MAP kinases, but suppressed AP-1 activity by inducing the expression of basic leucine zipper transcription factor, ATF-like (BATF), a negative regulator of AP-1 in RPMI8226 cells. By contrast, engagement of CD99 enhanced AP-1 activity and did not change the BATF expression in Jurkat cells. CD99 engagement reduced the proliferation of RPMI8226 cells and expression of cyclin 1 and 3. Overall, these results suggest novel CD99 functions in RPMI8226 cells.
Keyword
MeSH Terms
Figure
Reference
-
1. Levy R, Dilley J, Fox RI, Warnke R. A human thymus-leukemia antigen defined by hybridoma monoclonal antibodies. Proc Natl Acad Sci U S A. 1979; 76:6552–6556.
Article2. Dworzak MN, Fritsch G, Buchinger P, Fleischer C, Printz D, Zellner A, Schollhammer A, Steiner G, Ambros PF, Gadner H. Flow cytometric assessment of human MIC2 expression in bone marrow, thymus, and peripheral blood. Blood. 1994; 83:415–425.
Article3. Park CK, Shin YK, Kim TJ, Park SH, Ahn GH. High CD99 expression in memory T and B cells in reactive lymph nodes. J Korean Med Sci. 1999; 14:600–606.
Article4. Bernard G, Zoccola D, Deckert M, Breittmayer JP, Aussel C, Bernard A. The E2 molecule (CD99) specifically triggers homotypic aggregation of CD4+ CD8+ thymocytes. J Immunol. 1995; 154:26–32.5. Hahn JH, Kim MK, Choi EY, Kim SH, Sohn HW, Ham DI, Chung DH, Kim TJ, Lee WJ, Park CK, Ree HJ, Park SH. CD99 (MIC2) regulates the LFA-1/ICAM-1-mediated adhesion of lymphocytes, and its gene encodes both positive and negative regulators of cellular adhesion. J Immunol. 1997; 159:2250–2258.6. Kasinrerk W, Tokrasinwit N, Moonsom S, Stockinger H. CD99 monoclonal antibody induce homotypic adhesion of Jurkat cells through protein tyrosine kinase and protein kinase C-dependent pathway. Immunol Lett. 2000; 71:33–41.
Article7. Jung KC, Kim NH, Park WS, Park SH, Bae Y. The CD99 signal enhances Fas-mediated apoptosis in the human leukemic cell line, Jurkat. FEBS Lett. 2003; 554:478–484.
Article8. Pettersen RD, Bernard G, Olafsen MK, Pourtein M, Lie SO. CD99 signals caspase-independent T cell death. J Immunol. 2001; 166:4931–4942.
Article9. Bernard G, Breittmayer JP, de MM, Trampont P, Hofman P, Senik A, Bernard A. Apoptosis of immature thymocytes mediated by E2/CD99. J Immunol. 1997; 158:2543–2550.10. Waclavicek M, Majdic O, Stulnig T, Berger M, Sunder-Plassmann R, Zlabinger GJ, Baumruker T, Stockl J, Ebner C, Knapp W, Pickl WF. CD99 engagement on human peripheral blood T cells results in TCR/CD3-dependent cellular activation and allows for Th1-restricted cytokine production. J Immunol. 1998; 161:4671–4678.11. Yoon SS, Kim HJ, Chung DH, Kim TJ. CD99 costimulation up-regulates T cell receptor-mediated activation of JNK and AP-1. Mol Cells. 2004; 18:186–191.12. Hahn MJ, Yoon SS, Sohn HW, Song HG, Park SH, Kim TJ. Differential activation of MAP kinase family members triggered by CD99 engagement. FEBS Lett. 2000; 470:350–354.
Article13. Byun HJ, Hong IK, Kim E, Jin YJ, Jeoung DI, Hahn JH, Kim YM, Park SH, Lee H. A splice variant of CD99 increases motility and MMP-9 expression of human breast cancer cells through the AKT-, ERK-, and JNK-dependent AP-1 activation signaling pathways. J Biol Chem. 2006; 281:34833–34847.
Article14. Choi EY, Park WS, Jung KC, Kim SH, Kim YY, Lee WJ, Park SH. Engagement of CD99 induces up-regulation of TCR and MHC class I and II molecules on the surface of human thymocytes. J Immunol. 1998; 161:749–754.15. Husak Z, Printz D, Schumich A, Potschger U, Dworzak MN. Death induction by CD99 ligation in TEL/AML1-positive acute lymphoblastic leukemia and normal B cell precursors. J Leukoc Biol. 2010; 88:405–412.
Article16. Yoon SS, Jung KI, Choi YL, Choi EY, Lee IS, Park SH, Kim TJ. Engagement of CD99 triggers the exocytic transport of ganglioside GM1 and the reorganization of actin cytoskeleton. FEBS Lett. 2003; 540:217–222.
Article17. Williams KL, Nanda I, Lyons GE, Kuo CT, Schmid M, Leiden JM, Kaplan MH, Taparowsky EJ. Characterization of murine BATF: a negative regulator of activator protein-1 activity in the thymus. Eur J Immunol. 2001; 31:1620–1627.
Article18. Iacobelli M, Wachsman W, McGuire KL. Repression of IL-2 promoter activity by the novel basic leucine zipper p21SNFT protein. J Immunol. 2000; 165:860–868.
Article19. Echlin DR, Tae HJ, Mitin N, Taparowsky EJ. B-ATF functions as a negative regulator of AP-1 mediated transcription and blocks cellular transformation by Ras and Fos. Oncogene. 2000; 19:1752–1763.
Article20. Dorsey MJ, Tae HJ, Sollenberger KG, Mascarenhas NT, Johansen LM, Taparowsky EJ. B-ATF: a novel human bZIP protein that associates with members of the AP-1 transcription factor family. Oncogene. 1995; 11:2255–2265.21. Thornton TM, Zullo AJ, Williams KL, Taparowsky EJ. Direct manipulation of activator protein-1 controls thymocyte proliferation in vitro. Eur J Immunol. 2006; 36:160–169.
Article22. Liao J, Humphrey SE, Poston S, Taparowsky EJ. Batf promotes growth arrest and terminal differentiation of mouse myeloid leukemia cells. Mol Cancer Res. 2011; 9:350–363.
Article23. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001; 25:402–408.
Article24. Bernard G, Raimondi V, Alberti I, Pourtein M, Widjenes J, Ticchioni M, Bernard A. CD99 (E2) up-regulates alpha4beta1-dependent T cell adhesion to inflamed vascular endothelium under flow conditions. Eur J Immunol. 2000; 30:3061–3065.
Article25. Gil MC, Lee MH, Seo JI, Choi YL, Kim MK, Jung KC, Park SH, Kim TJ. Characterization and epitope mapping of two monoclonal antibodies against human CD99. Exp Mol Med. 2002; 34:411–418.
Article26. Shaulian E, Karin M. AP-1 in cell proliferation and survival. Oncogene. 2001; 20:2390–2400.
Article27. Hess J, Angel P, Schorpp-Kistner M. AP-1 subunits: quarrel and harmony among siblings. J Cell Sci. 2004; 117:5965–5973.
Article28. Matthews CP, Colburn NH, Young MR. AP-1 a target for cancer prevention. Curr Cancer Drug Targets. 2007; 7:317–324.
Article29. Senga T, Iwamoto T, Humphrey SE, Yokota T, Taparowsky EJ, Hamaguchi M. Stat3-dependent induction of BATF in M1 mouse myeloid leukemia cells. Oncogene. 2002; 21:8186–8191.
Article30. Su ZZ, Lee SG, Emdad L, Lebdeva IV, Gupta P, Valerie K, Sarkar D, Fisher PB. Cloning and characterization of SARI (suppressor of AP-1, regulated by IFN). Proc Natl Acad Sci U S A. 2008; 105:20906–20911.
Article31. Quigley M, Pereyra F, Nilsson B, Porichis F, Fonseca C, Eichbaum Q, Julg B, Jesneck JL, Brosnahan K, Imam S, Russell K, Toth I, Piechocka-Trocha A, Dolfi D, Angelosanto J, Crawford A, Shin H, Kwon DS, Zupkosky J, Francisco L, Freeman GJ, Wherry EJ, Kaufmann DE, Walker BD, Ebert B, Haining WN. Transcriptional analysis of HIV-specific CD8+ T cells shows that PD-1 inhibits T cell function by upregulating BATF. Nat Med. 2010; 16:1147–1151.
Article32. Liu Y, Lu C, Shen Q, Munoz-Medellin D, Kim H, Brown PH. AP-1 blockade in breast cancer cells causes cell cycle arrest by suppressing G1 cyclin expression and reducing cyclin-dependent kinase activity. Oncogene. 2004; 23:8238–8246.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The increased expression of CD99 in a differentiated neuroblastoma cell line
- The CXCR4 Antagonist AMD3100 Has Dual Effects on Survival and Proliferation of Myeloma Cells In Vitro
- Bortezomib inhibits the survival and proliferation of bone marrow stromal cells
- CD99 type II is a determining factor for the differentiation of primitive neuroectodermal cells
- A Case of Cutaneous Plasmacytoma Treated with Thalidomide