Immune Netw.
2013 Jun;13(3):102-106. 10.4110/in.2013.13.3.102.
Extravasating Neutrophil-derived Microparticles Preserve Vascular Barrier Function in Inflamed Tissue
- Affiliations
-
- 1Department of Microbiology and Immunology, David H. Smith Center for Vaccine Biology and Immunology, University of Rochester, Rochester, NY 14642, USA. young-min_hyun@urmc.rochester.edu
- 2Epithelial Pathobiology and Mucosal Inflammation Research Unit, Department of Pathology and Laboratory Medicine, Emory University School of Medicine, Atlanta, GA 30332, USA.
- KMID: 2150773
- DOI: http://doi.org/10.4110/in.2013.13.3.102
Abstract
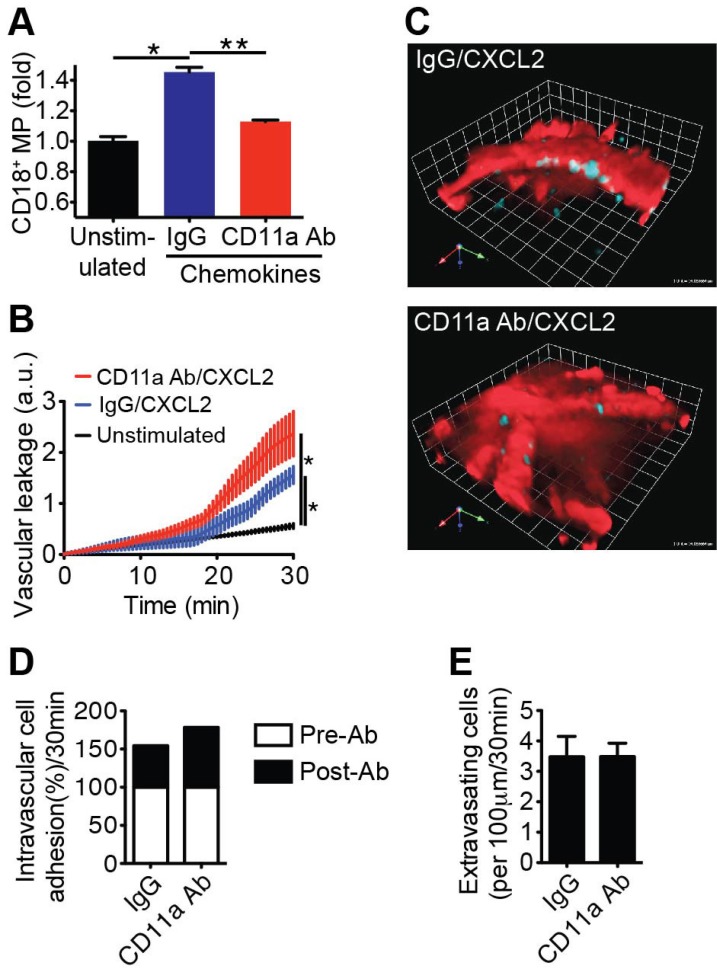
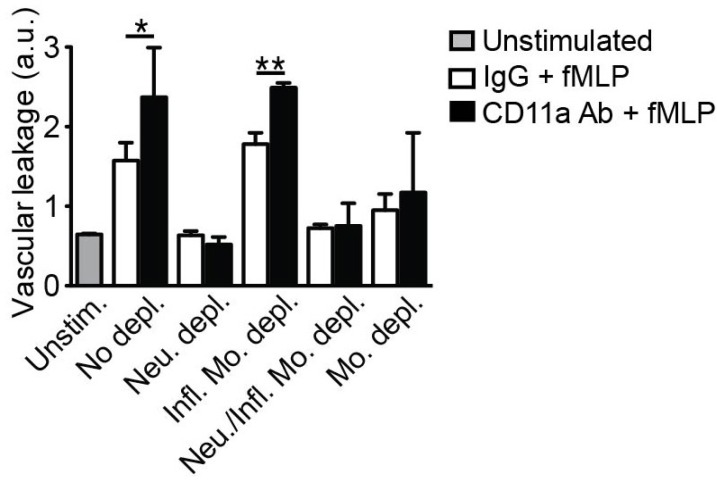
- Emerging evidence suggests that gap formation and opening of the endothelial junctions during leukocyte extravasation is actively controlled to maintain the integrity of the vascular barrier. While the role for endothelial cells to this process has been well defined, it is not clear whether leukocytes are also actively contributing to endothelial barrier function. We have recently showed that extravasating leukocytes deposit microparticles on the subendothelium during the late stages of extravasation, which is LFA-1 dependent. Using multiphotonintravital microscopy (MP-IVM) of mouse cremaster muscle vessels in the current work, we show that microparticle formation and deposition maintains the integrity of the microvascular barrier during leukocyte extravasation. Inhibition of neutrophil-derived microparticle formation resulted in dramatically increased vascular leakage. These findings suggest that deposition of microparticles during neutrophil extravasation is essential for maintaining endothelial barrier function and may result in temporal difference between neutrophil extravasation and an increase in vascular leakage.
MeSH Terms
Figure
Cited by 1 articles
-
Neutrophil Extravasation Cascade: What Can We Learn from Two-photon Intravital Imaging?
Sang A Park, Young-Min Hyun
Immune Netw. 2016;16(6):317-321. doi: 10.4110/in.2016.16.6.317.
Reference
-
1. Phillipson M, Heit B, Colarusso P, Liu L, Ballantyne CM, Kubes P. Intraluminal crawling of neutrophils to emigration sites: a molecularly distinct process from adhesion in the recruitment cascade. J Exp Med. 2006; 203:2569–2575. PMID: 17116736.
Article2. Shulman Z, Shinder V, Klein E, Grabovsky V, Yeger O, Geron E, Montresor A, Bolomini-Vittori M, Feigelson SW, Kirchhausen T, Laudanna C, Shakhar G, Alon R. Lymphocyte crawling and transendothelial migration require chemokine triggering of high-affinity LFA-1 integrin. Immunity. 2009; 30:384–396. PMID: 19268609.
Article3. Sumagin R, Prizant H, Lomakina E, Waugh RE, Sarelius IH. LFA-1 and Mac-1 define characteristically different intralumenal crawling and emigration patterns for monocytes and neutrophils in situ. J Immunol. 2010; 185:7057–7066. PMID: 21037096.
Article4. Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Lauvau CumG A, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007; 317:666–670. PMID: 17673663.
Article5. Hyun YM, Sumagin R, Sarangi PP, Lomakina E, Overstreet MG, Baker CM, Fowell DJ, Waugh RE, Sarelius IH, Kim M. Uropod elongation is a common final step in leukocyte extravasation through inflamed vessels. J Exp Med. 2012; 209:1349–1362. PMID: 22711877.
Article6. Sumagin R, Kuebel JM, Sarelius IH. Leukocyte rolling and adhesion both contribute to regulation of microvascular permeability to albumin via ligation of ICAM-1. Am J Physiol Cell Physiol. 2011; 301:C804–C813. PMID: 21653902.
Article7. Boring L, Gosling J, Chensue SW, Kunkel SL, Farese RV Jr, Broxmeyer HE, Charo IF. Impaired monocyte migration and reduced type 1 (Th1) cytokine re sponses in C-C chemokine receptor 2 knockout mice. J Clin Invest. 1997; 100:2552–2561. PMID: 9366570.8. Dalli J, Norling LV, Renshaw D, Cooper D, Leung KY, Perretti M. Annexin 1 mediates the rapid anti-inflammatory effects of neutrophil-derived microparticles. Blood. 2008; 112:2512–2519. PMID: 18594025.
Article9. Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008; 83:64–70. PMID: 17884993.
Article10. Soehnlein O, Zernecke A, Eriksson EE, Rothfuchs AG, Pham CT, Herwald H, Bidzhekov K, Rottenberg ME, Weber C, Lindbom L. Neutrophil secretion products pave the way for inflammatory monocytes. Blood. 2008; 112:1461–1471. PMID: 18490516.
Article11. Woodfin A, Voisin MB, Beyrau M, Colom B, Caille D, Diapouli FM, Nash GB, Chavakis T, Albelda SM, Rainger GE, Meda P, Imhof BA, Nourshargh S. The junctional adhesion molecule JAM-C regulates polarized transendothelial migration of neutrophils in vivo. Nat Immunol. 2011; 12:761–769. PMID: 21706006.
Article12. Kreisel D, Nava RG, Li W, Zinselmeyer BH, Wang B, Lai J, Pless R, Gelman AE, Krupnick AS, Miller MJ. In vivo two-photon imaging reveals monocyte-dependent neutrophil extravasation during pulmonary inflammation. Proc Natl Acad Sci U S A. 2010; 107:18073–18078. PMID: 20923880.
Article13. Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994; 174:83–93. PMID: 8083541.14. Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003; 19:71–82. PMID: 12871640.
Article15. Kim JV, Kang SS, Dustin ML, McGavern DB. Myelomonocytic cell recruitment causes fatal CNS vascular injury during acute viral meningitis. Nature. 2009; 457:191–195. PMID: 19011611.
Article16. Bjöork J, Hedqvist P, Arfors KE. Increase in vascular permeability induced by leukotriene B4 and the role of polymorphonuclear leukocytes. Inflammation. 1982; 6:189–200. PMID: 6179872.
Article17. Rosengren S, Ley K, Arfors KE. Dextran sulfate prevents LTB4-induced permeability increase, but not neutrophil emigration, in the hamster cheek pouch. Microvasc Res. 1989; 38:243–254. PMID: 2481803.
Article18. Wedmore CV, Williams TJ. Control of vascular permeability by polymorphonuclear leukocytes in inflammation. Nature. 1981; 289:646–650. PMID: 7464931.
Article19. Herwald H, Cramer H, Möorgelin M, Russell W, Sollenberg U, Norrby-Teglund A, Flodgaard H, Lindbom L, Bjöorck L. M protein, a classical bacterial virulence determinant, forms complexes with fibrinogen that induce vascular leakage. Cell. 2004; 116:367–379. PMID: 15016372.
Article20. Sekido N, Mukaida N, Harada A, Nakanishi I, Watanabe Y, Matsushima K. Prevention of lung reperfusion injury in rabbits by a monoclonal antibody against interleukin-8. Nature. 1993; 365:654–657. PMID: 8413628.
Article21. Petri B, Kaur J, Long EM, Li H, Parsons SA, Butz S, Phillipson M, Vestweber D, Patel KD, Robbins SM, Kubes P. Endothelial LSP1 is involved in endothelial dome formation, minimizing vascular permeability changes during neutrophil transmigration in vivo. Blood. 2011; 117:942–952. PMID: 21030556.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- NLRP3 Exacerbate NETosis-Associated Neuroinflammation in an LPS-Induced Inflamed Brain
- Extracellular Vesicles of Neutrophils
- Evaluation of Intestinal Epithelial Barrier Function in Inflammatory Bowel Diseases Using Murine Intestinal Organoids
- Association between vascular access failure and microparticles in hemodialysis patients
- Real-time observation of neutrophil extracellular trap formation in the inflamed mouse brain via two-photon intravital imaging