Immune Netw.
2011 Dec;11(6):358-363. 10.4110/in.2011.11.6.358.
Influence of Interferon-gamma Deficiency in Immune Tolerance Induced by Male Islet Transplantation
- Affiliations
-
- 1Department of Microbiology and Immunology, Seoul National University College of Medicine, Seoul 110-799, Korea. chgpark@snu.ac.kr
- 2Tumor Immunity Medical Research Center, Seoul National University College of Medicine, Seoul 110-799, Korea.
- 3Cancer Research Institute, Seoul National University College of Medicine, Seoul 110-799, Korea.
- 4Xenotransplantation Research Center, Seoul National University College of Medicine, Seoul 110-799, Korea.
- KMID: 2150720
- DOI: http://doi.org/10.4110/in.2011.11.6.358
Abstract
- BACKGROUND
Traditionally, interferon-gamma (IFN-gamma) was regarded as a pro-inflammatory cytokine, however, recent reports suggested role of IFN-gamma in immune tolerance. In our previous report, we could induce tolerance to male antigen (HY) just by male islet transplantation in wild type C57BL/6 mice without any immunological intervention. We tried to investigate the influence of IFN-gamma deficiency on tolerance induction by male islet transplantation.
METHODS
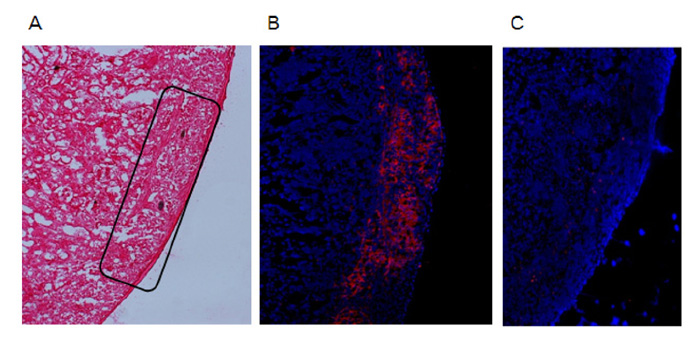
To examine the immunogenicity of male tissue in the absence of IFN-gamma, we transplanted male IFN-gamma knock-out (KO) skin to female IFN-gamma KO mice. Next, we analyzed male IFN-gamma KO islet to streptozotocin-induced diabetic female IFN-gamma KO mice. And, we checked the functionality of grafted islet by graft removal and insulin staining.
RESULTS
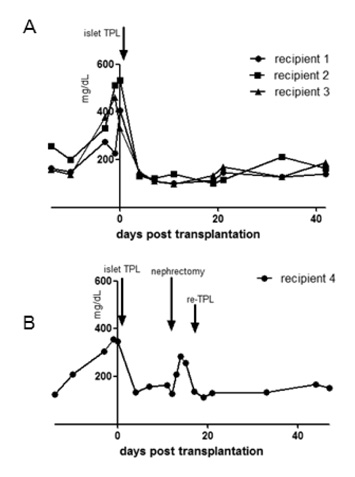
As our previous results in wild type C57BL/6 mice, female IFN-gamma KO mice rejected male IFN-gamma KO skin within 29 days, and did not reject male IFN-gamma KO islet. The maintenance of normal blood glucose level was dependent on the presence of grafted male islet. And the male islet recipient did not reject 2nd challenge of male islet graft also.
CONCLUSION
Deficiency of IFN-gamma does not have influence on the result of male skin graft and male islet transplantation. Conclusively, male islet transplantation induced T cell tolerance is not dependent on the presence of IFN-gamma.
MeSH Terms
Figure
Reference
-
1. Millrain M, Chandler P, Dazzi F, Scott D, Simpson E, Dyson PJ. Examination of HY response: T cell expansion, immunodominance, and cross-priming revealed by HY tetramer analysis. J Immunol. 2001. 167:3756–3764.
Article2. Simpson E, Scott D, Chandler P. The male-specific histocompatibility antigen, H-Y: a history of transplantation, immune response genes, sex determination and expression cloning. Annu Rev Immunol. 1997. 15:39–61.
Article3. Yoon IH, Choi SE, Kim YH, Yang SH, Park JH, Park CS, Kim Y, Kim JS, Kim SJ, Simpson E, Park CG. Pancreatic islets induce CD4(+) [corrected] CD25(-)Foxp3(+) [corrected] T-cell regulated tolerance to HY-mismatched skin grafts. Transplantation. 2008. 86:1352–1360.
Article4. Kelchtermans H, Billiau A, Matthys P. How interferon-gamma keeps autoimmune diseases in check. Trends Immunol. 2008. 29:479–486.5. Manoury-Schwartz B, Chiocchia G, Bessis N, Abehsira-Amar O, Batteux F, Muller S, Huang S, Boissier MC, Fournier C. High susceptibility to collagen-induced arthritis in mice lacking IFN-gamma receptors. J Immunol. 1997. 158:5501–5506.6. Vermeire K, Heremans H, Vandeputte M, Huang S, Billiau A, Matthys P. Accelerated collagen-induced arthritis in IFN-gamma receptor-deficient mice. J Immunol. 1997. 158:5507–5513.7. Billiau A, Heremans H, Vandekerckhove F, Dijkmans R, Sobis H, Meulepas E, Carton H. Enhancement of experimental allergic encephalomyelitis in mice by antibodies against IFN-gamma. J Immunol. 1988. 140:1506–1510.8. Caspi RR, Chan CC, Grubbs BG, Silver PB, Wiggert B, Parsa CF, Bahmanyar S, Billiau A, Heremans H. Endogenous systemic IFN-gamma has a protective role against ocular auto-immunity in mice. J Immunol. 1994. 152:890–899.9. Eriksson U, Kurrer MO, Sebald W, Brombacher F, Kopf M. Dual role of the IL-12/IFN-gamma axis in the development of autoimmune myocarditis: induction by IL-12 and protection by IFN-gamma. J Immunol. 2001. 167:5464–5469.
Article10. Ferber IA, Brocke S, Taylor-Edwards C, Ridgway W, Dinisco C, Steinman L, Dalton D, Fathman CG. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE). J Immunol. 1996. 156:5–7.11. Jones LS, Rizzo LV, Agarwal RK, Tarrant TK, Chan CC, Wiggert B, Caspi RR. IFN-gamma-deficient mice develop experimental autoimmune uveitis in the context of a deviant effector response. J Immunol. 1997. 158:5997–6005.12. Ring GH, Dai Z, Saleem S, Baddoura FK, Lakkis FG. Increased susceptibility to immunologically mediated glomerulonephritis in IFN-gamma-deficient mice. J Immunol. 1999. 163:2243–2248.13. Willenborg DO, Fordham S, Bernard CC, Cowden WB, Ramshaw IA. IFN-gamma plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. J Immunol. 1996. 157:3223–3227.14. Nishibori T, Tanabe Y, Su L, David M. Impaired development of CD4+ CD25+ regulatory T cells in the absence of STAT1: increased susceptibility to autoimmune disease. J Exp Med. 2004. 199:25–34.
Article15. Wang Z, Hong J, Sun W, Xu G, Li N, Chen X, Liu A, Xu L, Sun B, Zhang JZ. Role of IFN-gamma in induction of Foxp3 and conversion of CD4+ CD25-T cells to CD4+ Tregs. J Clin Invest. 2006. 116:2434–2441.16. Hong J, Li N, Zhang X, Zheng B, Zhang JZ. Induction of CD4+CD25+ regulatory T cells by copolymer-I through activation of transcription factor Foxp3. Proc Natl Acad Sci U S A. 2005. 102:6449–6454.
Article17. Feng G, Gao W, Strom TB, Oukka M, Francis RS, Wood KJ, Bushell A. Exogenous IFN-gamma ex vivo shapes the alloreactive T-cell repertoire by inhibition of Th17 responses and generation of functional Foxp3+ regulatory T cells. Eur J Immunol. 2008. 38:2512–2527.
Article18. Sawitzki B, Kingsley CI, Oliveira V, Karim M, Herber M, Wood KJ. IFN-gamma production by alloantigen-reactive regulatory T cells is important for their regulatory function in vivo. J Exp Med. 2005. 201:1925–1935.
Article19. Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, Belladonna ML, Fioretti MC, Alegre ML, Puccetti P. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003. 4:1206–1212.
Article20. Moffett JR, Namboodiri MA. Tryptophan and the immune response. Immunol Cell Biol. 2003. 81:247–265.
Article21. Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004. 4:762–774.
Article22. Nast CC, Zuo XJ, Prehn J, Danovitch GM, Wilkinson A, Jordan SC. Gamma-interferon gene expression in human renal allograft fine-needle aspirates. Transplantation. 1994. 57:498–502.
Article23. Cobbold SP, Castejon R, Adams E, Zelenika D, Graca L, Humm S, Waldmann H. Induction of foxP3+ regulatory T cells in the periphery of T cell receptor transgenic mice tolerized to transplants. J Immunol. 2004. 172:6003–6010.
Article24. Lechler RI, Garden OA, Turka LA. The complementary roles of deletion and regulation in transplantation tolerance. Nat Rev Immunol. 2003. 3:147–158.
Article25. Li XC, Strom TB, Turka LA, Wells AD. T cell death and transplantation tolerance. Immunity. 2001. 14:407–416.
Article26. Scully R, Qin S, Cobbold S, Waldmann H. Mechanisms in CD4 antibody-mediated transplantation tolerance: kinetics of induction, antigen dependency and role of regulatory T cells. Eur J Immunol. 1994. 24:2383–2392.
Article27. Waldmann H, Cobbold S. Regulating the immune response to transplants. A role for CD4+ regulatory cells? Immunity. 2001. 14:399–406.28. Zhang L, Yi H, Xia XP, Zhao Y. Transforming growth factor-beta: an important role in CD4+CD25+ regulatory T cells and immune tolerance. Autoimmunity. 2006. 39:269–276.
Article29. Tanaka K, Albin MJ, Yuan X, Yamaura K, Habicht A, Murayama T, Grimm M, Waaga AM, Ueno T, Padera RF, Yagita H, Azuma M, Shin T, Blazar BR, Rothstein DM, Sayegh MH, Najafian N. PDL1 is required for peripheral transplantation tolerance and protection from chronic allograft rejection. J Immunol. 2007. 179:5204–5210.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immune Tolerance in Murine Islet Transplantation Across HY Disparity
- Partial Interferon-gamma Receptor Deficiency in Patients with Disseminated Tuberculosis
- Induction of Immune Tolerance after Transplantation
- Enhancement of Allergen-induced Airway Inflammation by NOX2 Deficiency
- Effect of Interleukin-10 on Lipopolysaccahride/Interferon-gamma- Induced Chemokine Mig Gene Expression