Immune Netw.
2011 Apr;11(2):100-106. 10.4110/in.2011.11.2.100.
Characterization of CTL Clones Specific for Single Antigen, H60 Minor Histocompatibility Antigen
- Affiliations
-
- 1Department of Biomedical Sciences, Seoul National University College of Medicine, Seoul 110-799, Korea. eycii@snu.ac.kr
- 2Graduate Program of Immunology, Seoul National University College of Medicine, Seoul 110-799, Korea.
- 3Division of Life and Pharmaceutical Sciences, Ewha Womans University, Seoul 120-750, Korea.
- KMID: 2150697
- DOI: http://doi.org/10.4110/in.2011.11.2.100
Abstract
- BACKGROUND
Disparities of Minor H antigens can induce graft rejection after MHC-matched transplantation. H60 has been characterized as a dominant antigen expressed on hematopoietic cells and considered to be an ideal model antigen for study on graft-versus-leukemia effect.
METHODS
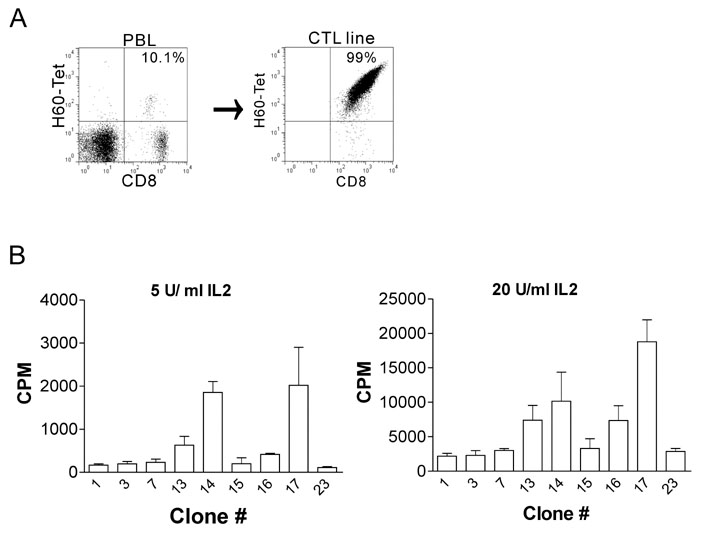
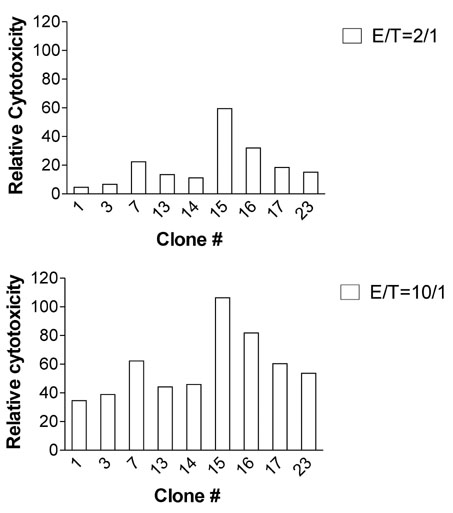
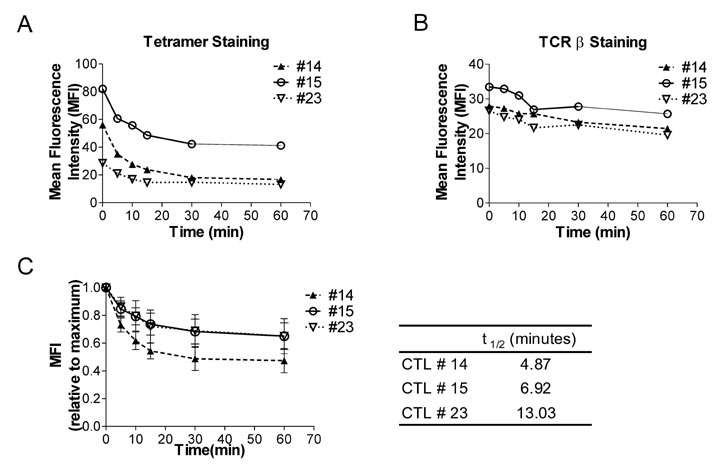
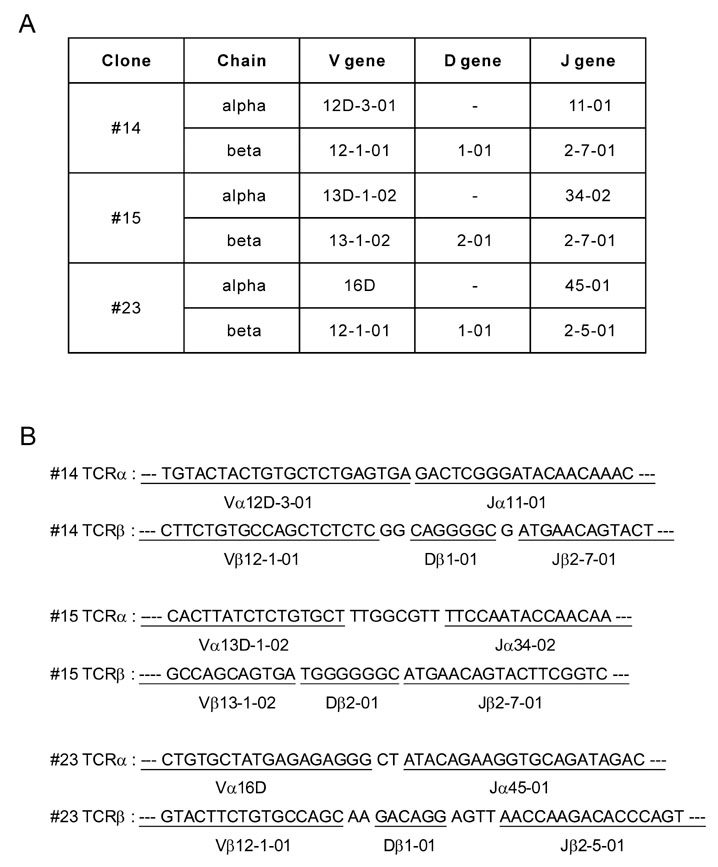
Splenocytes from C57BL/6 mice immunized with H60 congenic splenocytes were used for establishment of H60-specific CTL clones. Then the clones were characterized for proliferation capacity and cytotoxicity after stimulation with H60. Clone #14, #15, and #23 were tested for the TCR binding avidity to H60-peptide/H-2Kb and analyzed for TCR sequences.
RESULTS
H60-specific CTL clones showed different levels of proliferation capacity and cytotoxic activity to H60-stimulation. Clones #14, #15, and #23 showed high proliferation activity, high cytotoxicity, and low activities on both aspects, respectively, and have TCRs with different binding avidities to H60-peptide/H-2Kb with t 1/2 values of 4.87, 6.92, and 13.03 minutes, respectively. The TCR usages were Valpha12D-3-01+Jalpha11-01 and Vbeta12-1-01+Dbeta1-01+J2-7-01 for clone #14, Valpha13D-1-02+Jalpha34-02 and Vbeta13-1-02+Dbeta2-01+Jbeta2-7-01 for clone #15, and Valpha16D+Jalpha45-01 and Vbeta12-1-01+Dbeta1-01+Jbeta2-5-01 for clone #23.
CONCLUSION
The results will be useful for modeling GVL and generation TCR transgenic mouse.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Skewed Dendritic Cell Differentiation of MyD88-Deficient Donor Bone Marrow Cells, Instead of Massive Expansion as Myeloid-Derived Suppressor Cells, Aggravates GVHD
Young-Kwan Lee, Ji-Min Ju, Woo-Jeong Shon, Sehwa Oh, Chang-Ki Min, Myung-Soo Kang, Dong-Mi Shin, Eun Young Choi
Immune Netw. 2018;18(6):. doi: 10.4110/in.2018.18.e44.Selection of Thymocytes Expressing Transgenic TCR Specific for a Minor Histocompatibility Antigen, H60
Ji-Min Ju, Min Bum Kim, Su Jeong Ryu, Joo Young Kim, Jun Chang, Eun Young Choi
Immune Netw. 2015;15(5):222-231. doi: 10.4110/in.2015.15.5.222.
Reference
-
1. Simpson E. Minor histocompatibility antigens. Immunol Lett. 1991. 29:9–14.
Article2. Simpson E, Roopenian D. Minor histocompatibility antigens. Curr Opin Immunol. 1997. 9:655–661.
Article3. Wettstein PJ. Immunodominance in the T-cell response to multiple non-H-2 histocompatibility antigens. II. Observation of a hierarchy among dominant antigens. Immunogenetics. 1986. 24:24–31.
Article4. Choi EY, Yoshimura Y, Christianson GJ, Sproule TJ, Malarkannan S, Shastri N, Joyce S, Roopenian DC. Quantitative analysis of the immune response to mouse non-MHC transplantation antigens in vivo: the H60 histocompatibility antigen dominates over all others. J Immunol. 2001. 166:4370–4379.
Article5. Malarkannan S, Shih PP, Eden PA, Horng T, Zuberi AR, Christianson G, Roopenian D, Shastri N. The molecular and functional characterization of a dominant minor H antigen, H60. J Immunol. 1998. 161:3501–3509.6. Korngold R. Lethal graft-versus-host disease in mice directed to multiple minor histocompatibility antigens: features of CD8+ and CD4+ T cell responses. Bone Marrow Transplant. 1992. 9:355–364.7. van Lochem E, de Gast B, Goulmy E. In vitro separation of host specific graft-versus-host and graft-versus-leukemia cytotoxic T cell activities. Bone Marrow Transplant. 1992. 10:181–183.8. Goulmy E. Human minor histocompatibility antigens: new concepts for marrow transplantation and adoptive immunotherapy. Immunol Rev. 1997. 157:125–140.
Article9. Choi JH, Yoon H, Min CK, Choi EY. Effects of Pre-conditioning Dose on the Immune Kinetics and Cytokine Production in the Leukocytes Infiltrating GVHD Tissues after MHC-matched Transplantation. Immune Netw. 2011. 11:68–78.
Article10. Ryu SJ, Jung KM, Yoo HS, Kim TW, Kim S, Chang J, Choi EY. Cognate CD4 help is essential for the reactivation and expansion of CD8 memory T cells directed against the hematopoietic cell-specific dominant minor histocompatibility antigen, H60. Blood. 2009. 113:4273–4280.
Article11. Choi EY, Christianson GJ, Yoshimura Y, Sproule TJ, Jung N, Joyce S, Roopenian DC. Immunodominance of H60 is caused by an abnormally high precursor T cell pool directed against its unique minor histocompatibility antigen peptide. Immunity. 2002. 17:593–603.
Article12. Choi JH, Ryu SJ, Jung KM, Kim S, Chang J, Kim TW, Choi EY. TCR diversity of H60-specific CD8 T cells during the response evolution and influence of CD4 help. Transplantation. 2009. 87:1609–1616.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Selection of Thymocytes Expressing Transgenic TCR Specific for a Minor Histocompatibility Antigen, H60
- Role for CD40 and CD40L Expression in Generating CD8 T Cell Response to Minor Histcompatibility Antigen, H60
- Subdominant H60 antigen-specific CD8 T-cell response precedes dominant H4 antigen-specific response during the initial phase of allogenic skin graft rejection
- Requirement of CD4 Help for Induction of CD8 T Cell Response Specific for Virally Derived H60
- Characterization of Dermatophagoides pteronyssinus-specific T cell clones from bronchial asthmatics