Transl Clin Pharmacol.
2015 Jun;23(1):21-25. 10.12793/tcp.2015.23.1.21.
Bioequivalence of the pharmacokinetics between two formulations of 0.2 mg tamsulosin hydrochloride in healthy subjects
- Affiliations
-
- 1Department of Clinical Pharmacology and Therapeutics, Seoul National University Hospital and College of Medicine, Seoul 110-744, Korea.
- 2Department of Clinical Pharmacology and Therapeutics, Kyung Hee University College of Medicine and Hospital, Seoul 130-872, Korea. bhkim98@khu.ac.kr
- KMID: 2148855
- DOI: http://doi.org/10.12793/tcp.2015.23.1.21
Abstract
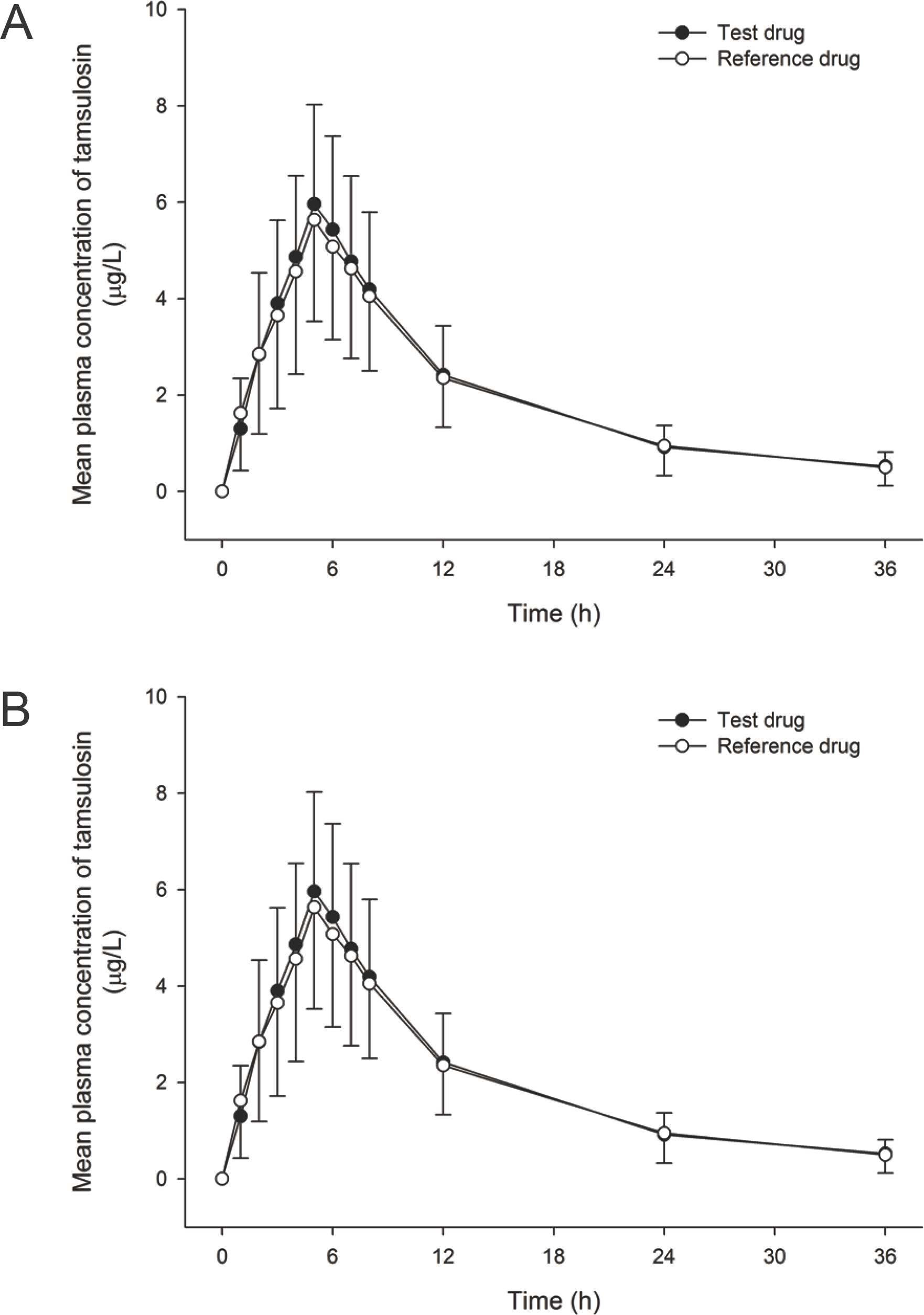
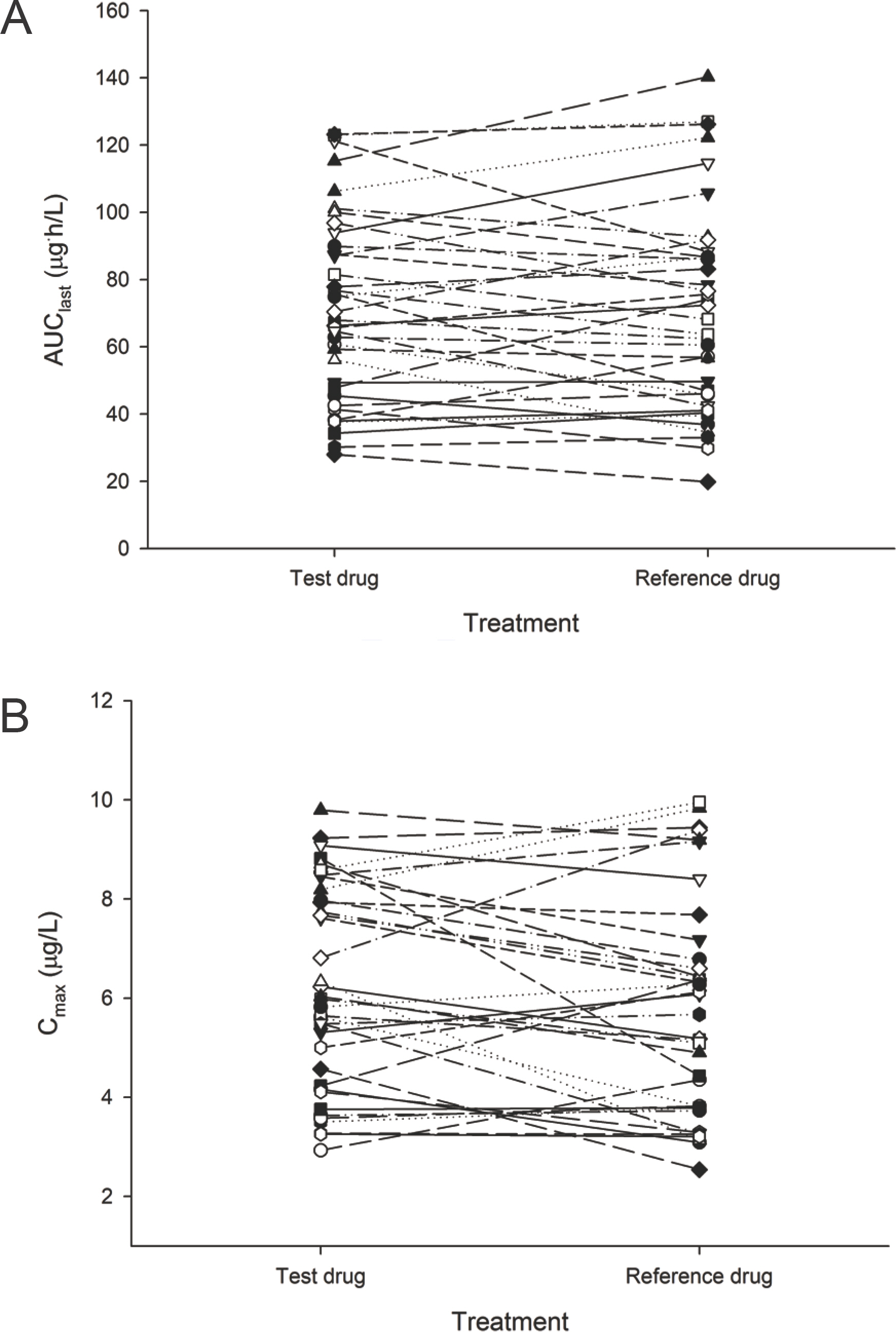
- Tamsulosin is an effective therapeutic option for lower urinary tract symptoms, as it selectively blocks alpha1A- and alpha1D-adrenoceptors in the bladder and prostate. The purpose of this study was to evaluate the bioequivalence in the pharmacokinetics (PK) of two 0.2 mg tamsulosin formulations when administered as the reference formulation (Yuropa(R) sustained-release tablet) vs. the test formulation (Yutanal(R) capsule) in healthy male subjects. A randomized, open-label, single-dose, two-way, two-period, crossover study was conducted in 37 healthy volunteers. The 0.2 mg of tamsulosin as the test or the reference formulation were administered during each period, and serial blood samples were collected up to 36 hours after dosing for PK analyses. A non-compartmental analysis was used to estimate the PK parameters. Geometric mean ratios (GMR) and 90% confidence inter-vals (CIs) were calculated for the two formulations to compare the maximum concentration (Cmax) and the area under the concentration-time curve from time zero to the time of the last quantifiable concentration (AUClast). The mean Cmax and AUClast for the test formulation were 6.19 microg/L and 71.30 microg.h/L, respectively, and 5.76 microg/L and 70.38 microg.h /L for the reference formulation, respectively. The GMRs (90% CIs) of the Cmax and AUClast between the two formulations were 1.09 (1.01-1.17) and 1.03 (0.96-1.10), respectively. Tamsulosin 0.2 mg as the test formulation exhibited bioequivalent PK profiles to those of the reference formulation. Therefore, the test formulation is expected to be an alternative to the reference formulation without concerns about differences in drug exposure.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003; 61:37–49. doi: 10.1016/S0090-4295 (02)02243-4.
Article2. Lyseng-Williamson KA, Jarvis B, Wagstaff AJ. Tamsulosin: an update of its role in the management of lower urinary tract symptoms. Drugs. 2002; 62:135–167. doi: 10.2165/00003495-200262010-00006.3. Irwin DE, Kopp ZS, Agatep B, Milsom I, Abrams P. Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction. BJU Int. 2011; 108:1132–1138. doi: 10.1111/j.1464-410X.2010.09993.x.
Article4. Irwin DE, Milsom I, Kopp Z, Abrams P, Artibani W, Herschorn S. Prevalence, Severity, and symptom bother of lower urinary tract symptoms among men in the EPIC study: impact of overactive bladder. Eur Urol. 2009; 56:14–20. doi: 10.1016/j.eururo.2009.02.026.
Article5. Kannan H, Radican L, Turpin RS, Bolge SC. Burden of illness associated with lower urinary tract symptoms including overactive bladder/urinary incontinence. Urology. 2009; 74:34–38.
Article6. Andersson K-E. Mode of action of α-adrenoceptor antagonists in the treatment of lower urinary 225 tract symptoms. Current Prostate Reports. 2005; 3:28–33. doi: 10.1007/s11918-996-0012-1.7. Schwinn DA, Michelotti GA. α1-adrenergic receptors in the lower urinary tract and vascular bed: potential role for the α1d subtype in filling symptoms and effects of ageing on vascular expression. BJU Int. 2000; 85(Suppl 2):6–11. doi: 10.1046/j.1464-410X.2000.00061.x.8. http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020579s027lbl.pdfAccessedMay25. 2015.9. O'Leary MP. Tamsulosin: current clinical experience. Urology. 2001; 58(Suppl 1):42–48.10. Lee K, Choi S, Jeon H, Lee B, Kim H, Lee J, et al. Controlled release of tamsulosin from enteric coated sustained-release matrices with aqueous microchannels. J Kor Pharm Sci. 2004; 34:471–475.11. Wilde M, McTavish D. Tamsulosin. A review of its pharmacological properties and therapeutic potential in the management of symptomatic benign prostatic hyperplasia. Drugs. 1996; 52:883–898. doi: 10.2165/00003495-199652060-00012.12. Kim MS, Kim JS, You YH, Park HJ, Lee S, Park JS, et al. Development and 236 optimization of a novel oral controlled delivery system for tamsulosin hydrochloride using response surface methodology. Int J Pharm. 2007; 341:97–104.13. Kamimura H, Oishi S, Matsushima H, Watanabe T, Higuchi S, Hall M, et al. Identification of cytochrome P450 isozymes involved in metabolism of the α1-adrenoceptor blocker tamsulosin in human liver microsomes. Xenobiotica. 1998; 28:909–922.14. Ding L, Li L, Tao P, Yang J, Zhang Z. Quantitation of tamsulosin in human plasma by liquid chromatography–electrospray ionization mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2002; 767:75–81.
Article15. Prasaja B, Harahap Y, Lusthom W, Setiawan EC, Ginting MB, Hardiyanti , et al. A bioequivalence study of two tamsulosin sustained-release tablets in Indonesian healthy volunteers. Eur J Drug Metab Pharmacokinet. 2011; 36:109–113.
Article16. Nithiyananthan T, Shankarananth V, Rajasekhar K, Hareesh G, Kumar PN, Reddy RSP. Formulation and evaluation of tamsulosin hydrochloride as sustained release matrix tablet. Int J Chem Tech Res. 2009; 1:1278–1290.17. Matsushima H, Kamimura H, Soeishi Y, Watanabe T, Higuchi S, Tsunoo M. Pharmacokinetics and plasma protein binding of tamsulosin hydrochloride in rats, dogs, and humans. Drug Metab Dispos. 1998; 26:240–245.18. Franco-Salinas G, dze la Rosette JJ, Michel MC. Pharmacokinetics and pharmacodynamics of tamsulosin in its modified-release and oral controlled absorption system formulations. Clin Pharmacokinet. 2010; 49:177–188.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pharmacokinetic properties and bioequivalence of gefitinib 250 mg in healthy Korean male subjects
- Bioequivalence study of Donepezil hydrochloride in healthy Korean volunteers
- Evaluation of the pharmacokinetics and food effects of a novel formulation tamsulosin 0.4 mg capsule compared with a 0.2 mg capsule in healthy male volunteers
- Comparative pharmacokinetic and tolerability evaluation of two simvastatin 20 mg formulations in healthy Korean male volunteers
- Steady-State Pharmacokinetic Properties of Tamsulosin in Healthy Male Volunteers