J Korean Soc Clin Pharmacol Ther.
2013 Dec;21(2):130-140. 10.12793/jkscpt.2013.21.2.130.
Steady-State Pharmacokinetic Properties of Tamsulosin in Healthy Male Volunteers
- Affiliations
-
- 1Department of Clinical Trial Center, Kyungpook National University Hospital, Daegu, Korea. yry@knu.ac.kr
- 2Department of Biomedical Science, Kyungpook National University Graduate School, Daegu, Korea.
- 3KNU Bio-Medical Convergence Program for Creative Talent, Kyungpook National University Graduate School, Daegu, Korea.
- 4College of Pharmacy, Yeungnam University, Gyeongsan, Korea.
- 5School of Nursing, Yeungnam College of Science & Technology, Daegu, Korea.
- KMID: 2203226
- DOI: http://doi.org/10.12793/jkscpt.2013.21.2.130
Abstract
- BACKGROUND
To evaluate the pharmacokinetic properties of daily oral doses of tamsulosin administered to fasted healthy Korean male volunteers for 5 days.
METHODS
In a randomized, open-label, multiple-dose, two-period, crossover study, all 44 subjects were randomly assigned in a 1:1 ratio to receive a newly developed generic capsule formulation (test) or a branded capsule formulation (reference) of tamsulosin 0.2 mg, followed by a 10-day washout period and administration of the other formulation. Plasma concentrations of tamsulosin were assessed after administration of five-day multiple doses, using HPLC-MS/MS. Clinical and laboratory adverse events (AE) were assessed.
RESULTS
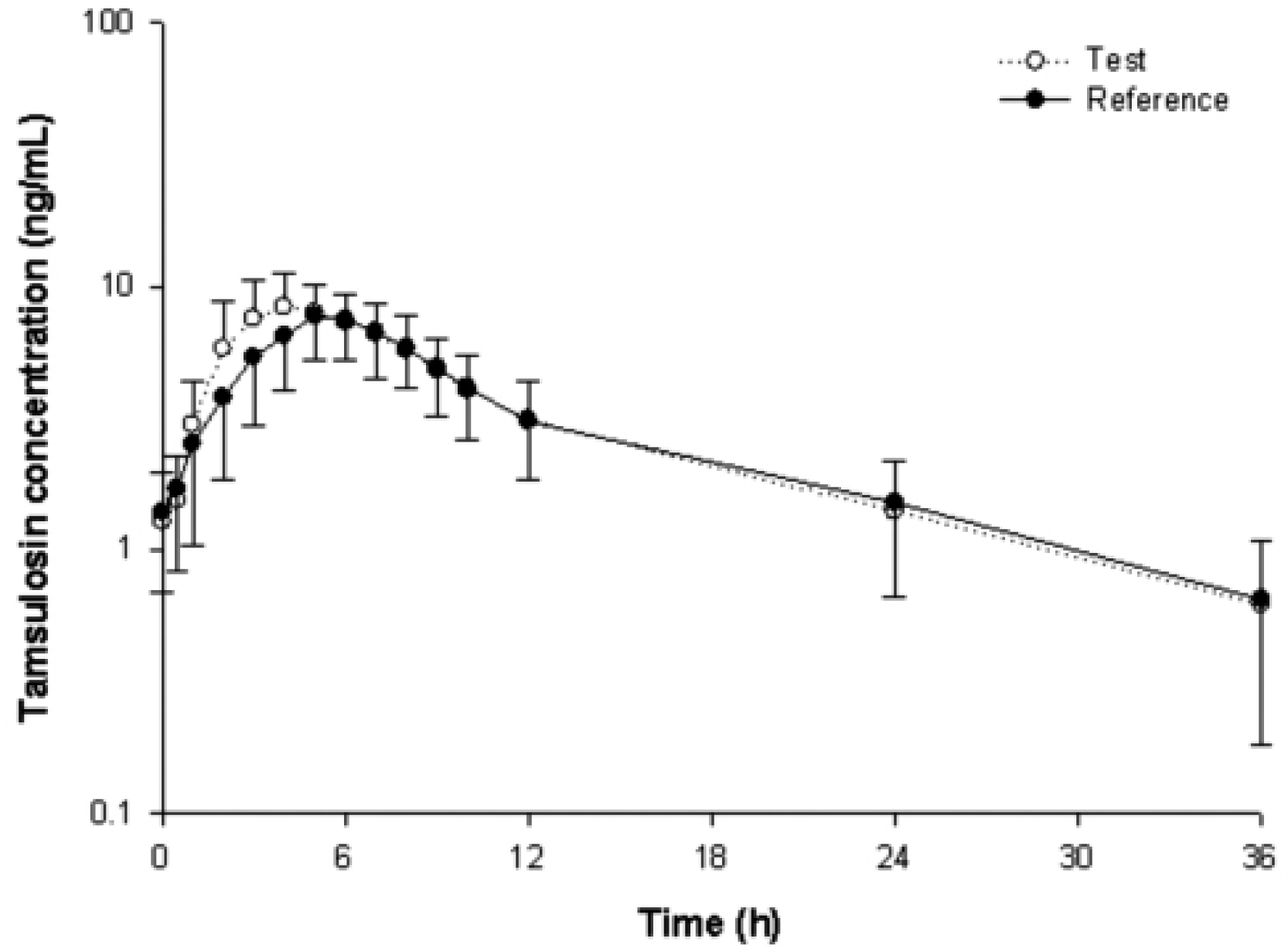
The mean (SD) pharmacokinetic properties with the test and reference formulations were as follows: Css,max, 9.0 (2.9) and 8.4 (2.6) ng/mL, respectively; median (range) tmax, 4 (2-6) and 5 (2-7) hours; AUCtau, 93.7 (31.5) and 88.2 (29.3) ng x h/mL; and t(1/2), 9.5 (2.6) and 10.0 (2.7) hours. The volume of distribution and clearance after oral administration of tamsulosin were 0.5 L/kg, and 0.04 L/h/kg, respectively. The accumulation ratios for 0.2 mg once-daily dosing regimen were 1.2. The 90% CIs of the geometric mean ratios for the log-transformed AUCtau (1.005-1.131) and Css,max (1.000-1.136) values were within the acceptable range for bioequivalence. No serious AE was reported during the study. Both formulations were well tolerated.
CONCLUSION
The results demonstrate that the Css,max and AUCtau values in the fasted subjects were higher than those in the fed from other study, with a shorter tmax values.
MeSH Terms
Figure
Reference
-
1. Logie J, Clifford GM, Farmer RDT. Incidence, prevalence and management of lower urinary tract symptoms in men in the UK. BJU Int. 2005; 95(4):557–562.
Article2. Glasser DB, Carson C 3rd, Kang JH, Laumann EO. Prevalence of storage and voiding symptoms among men aged 40 years and older in a US population-based study: results from the Male Attitudes Regarding Sexual Health study. Int J Clin Pract. 2007; 61(8):1294–1300.3. Andersson KE. Alpha-adrenoceptors and benign prostatic hyperplasia: basic principles for treatment with alpha-adrenoceptor antagonists. World J Urol. 2002; 19(6):390–396.4. Abrams P, Andersson KE. Muscarinic receptor antagonists for overactive bladder. BJU Int. 2007; 100(5):987–1006.
Article5. Naslund MJ, Miner M. A review of the clinical efficacy and safety of 5α-reductase inhibitors for the enlarged prostate. Clin Ther. 2007; 29(1):17–25.
Article6. van Hoogdaelm EJ, Soeishi Y, Matsushima H, Hiquchi S. Disposition of the selective α 1A-adrenoreceptor antagonist tamsulosin in humans: comparison with data from interspecies scaling. J Pharm Sci. 1997; 86(10):1156–1161.7. Michel MC, Grübbel B, Taguchi K, VerfÜrth F, Otto T, KrÖpfl D. Drugs for treatment of benign prostatic hyperplasia: affinity comparison at cloned alpha 1-adrenoceptor subtypes and in human prostate. J Auton Pharmacol. 1996; 16(1):21–28.8. Prasaja B, Harahap Y, Lusthom W, Seitiawan EC, Ginting MB, Hardiyanti , Lipin . A bioequivalence study of two tamsulosin sustained-release tablets in Indonesian healthy volunteers. Eur J Drug Metab Pharmacokinet. 2011; 36(2):109–113.
Article9. Lyseng-Williamson KA, Jarvis B, Wagstaff AJ. Tamsulosin: an update of its role in the management of lower urinary tract symptoms. Drugs. 2002; 62(1):135–167.10. Franco-Salinas G, de la Rosette JJMCH, Michel MC. Pharmacokinetics and pharmacodynamics of tamsulosin in its modified-release and oral controlled absorption system formulations. Clin Pharmacokinet. 2010; 49(3):177–188.
Article11. Koiso K, Akaza H, Kikuchi K, Aoyagi K, Ohba S, Miyazaki M, Ito M, Sueyoshi T, Matsushima H, Kamimura H, Watanabe T, Higuchi S. Pharmacokinetics of tamsulosin hydrochloride in patients with renal impairment: effects of α1-acid glycoprotein. J Clin Pharmacol. 1996; 36(11):1029–1038.12. Matsushima H, Kamimura H, Soeishi Y, Watanabe T, Higuchi S, Tsunoo M. Pharmacokinetics and plasma protein binding of tamsulosin hydrochloride in rats, dogs, and humans. Drug Metab Dispos. 1998; 26(3):240–245.13. Kamimura H, Oishi S, Matsushima H, Watanabe T, Higuchi S, Hall M, Wood SG, Chasseaud LF. Identification of cytochrome P450 isozymes involved in metabolism of the α1-adrenoceptor blocker tamsulosin in human liver microsomes. Xenobiotica. 1998; 8(10):909–922.14. Taguchi K, Saitoh M, Sato S, Asano M, Michel MC. Effects of tamsulosin metabolites at alpha-1 adrenoceptor subtypes. J Pharmacol Exp Ther. 1997; 280(1):1–5.15. Wolzt M, Fabrizii V, Dorner GT, Zanaschka G, Leufkens P, Krauwinkel WJ, Eichler HG. Pharmacokinetics of tamsulosin in subjects with normal and varying degrees of impaired renal function: an open-label single-dose and multiple-dose study. Eur J Clin Pharmacol. 1998; 54(4):367–373.
Article16. Miyazawa Y, Blum RA, Schentag JJ, Kamimura H, Matsushima H, Swarz H, Ito Y. Pharmacokinetics and safety of tamsulosin in subjects with normal and impaired renal or hepatic function. Curr Ther Res. 2001; 62(9):603–621.
Article17. Taguchi K, Schäfers RF, Michel MC. Radio-receptor assay analysis of tamsulosin and terazosin pharmacokinetics. Br J Clin Pharmacol. 1998; 45(1):49–55.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of the pharmacokinetics and food effects of a novel formulation tamsulosin 0.4 mg capsule compared with a 0.2 mg capsule in healthy male volunteers
- Serious adverse events during clinical trial for pharmacokinetic interaction between telmisartan and chlorthalidone in healthy Korean subjects: A case report
- Pharmacokinetics and Safety of Levodropropizine Controlled Release Tablet after Repeated Dosing in Healthy Male Volunteers
- Effects of JOINS® on the pharmacokinetic profiles of aceclofenac in healthy Korean volunteers: an open-label, multiple-dose, one sequence, two-period study
- Bioequivalence of the pharmacokinetics between two formulations of 0.2 mg tamsulosin hydrochloride in healthy subjects