J Korean Ophthalmol Soc.
2015 Dec;56(12):1893-1898. 10.3341/jkos.2015.56.12.1893.
Choroidal Thickness at the Outside of Fovea in Diabetic Retinopathy Using Spectral-Domain Optical Coherence Tomography
- Affiliations
-
- 1Department of Ophthalmology, Sanggye Paik Hospital, Inje University College of Medicine, Seoul, Korea. 991027js@hanmail.net
- KMID: 2148751
- DOI: http://doi.org/10.3341/jkos.2015.56.12.1893
Abstract
- PURPOSE
To evaluate choroidal thickness at the outside of the fovea in patients with diabetic retinopathy using spectral-domain optical coherence tomography.
METHODS
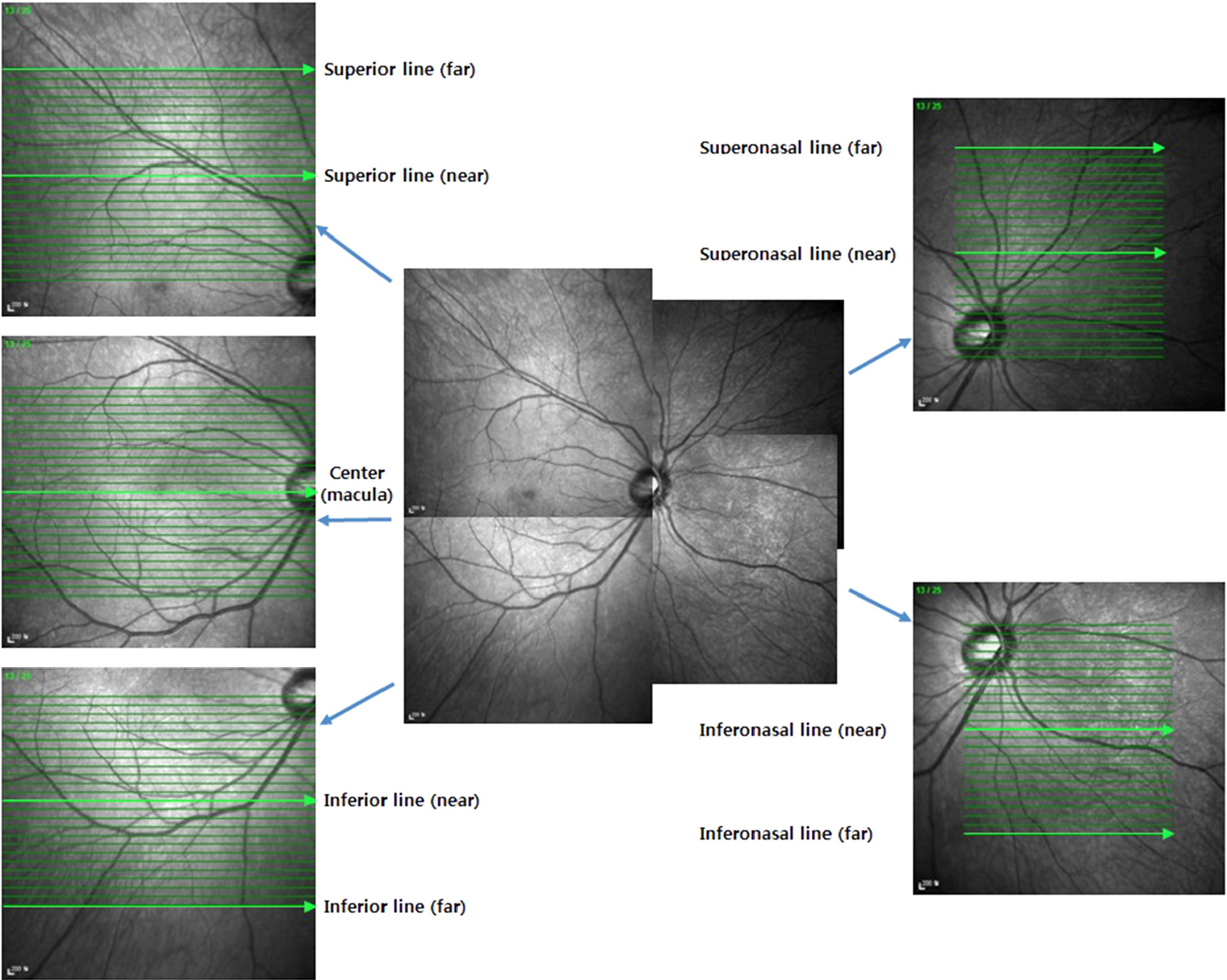
We examined 87 eyes of 87 patients with diabetic retinopathy and 40 eyes of 40 normal patients. Patients with diabetic retinopathy were divided into 3 groups according to the grade of diabetic retinopathy and macular edema. The choroidal thickness was obtained at the fovea and outside of the fovea using enhanced depth imaging of Spectralis optical coherence tomography. One foveal and 8 peripheral images were selected and choroidal thickness was measured from the outer border of the retinal pigment epithelium to the inner scleral border.
RESULTS
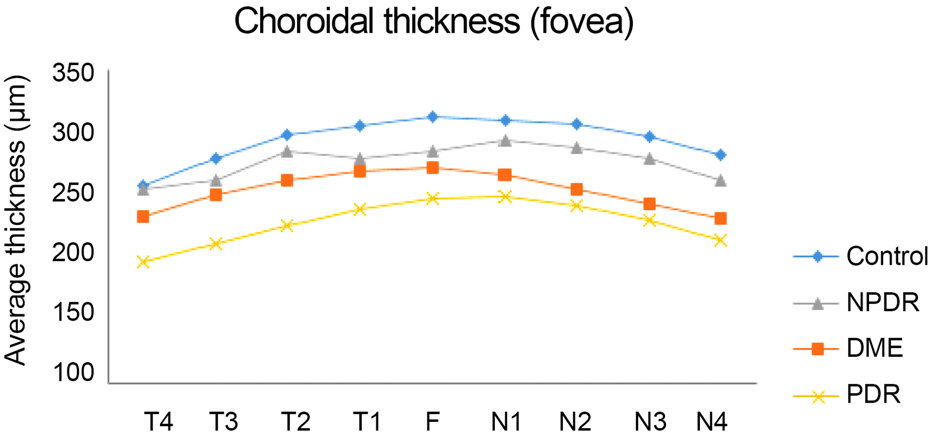
Subfoveal choroidal thickness was thinner with increasing severity of diabetic retinopathy. However, there was no significant difference between groups without the nasal side of the fovea. A statistically significant difference was observed over the fovea at the superotemporal area.
CONCLUSIONS
The choroidal thickness outside of the fovea was thinner with the severity of diabetic retinopathy and was more pronounced in the superotemporal area.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Antonetti DA, Barber AJ, Bronson SK. . Diabetic retinopathy: seeing beyond glucose-induced microvascular disease. Diabetes. 2006; 55:2401–11.2. Hidayat AA, Fine BS. Diabetic choroidopathy. Light and electron microscopic observations of seven cases. Ophthalmology. 1985; 92:512–22.3. Weinberger D, Kramer M, Priel E. . Indocyanine green angio-graphic findings in nonproliferative diabetic retinopathy. Am J Ophthalmol. 1998; 126:238–47.
Article4. Shiragami C, Shiraga F, Matsuo T. . Risk factors for diabetic choroidopathy in patients with diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2002; 240:436–42.
Article5. Cao J, McLeod S, Merges CA, Lutty GA. Choriocapillaris degen-eration and related pathologic changes in human diabetic eyes. Arch Ophthalmol. 1998; 116:589–97.
Article6. Coleman DJ, Silverman RH, Chabi A. . High-resolution ultra-sonic imaging of the posterior segment. Ophthalmology. 2004; 111:1344–51.
Article7. Bartsch DU, Weinreb RN, Zinser G, Freeman WR. Confocal scan-ning infrared laser ophthalmoscopy for indocyanine green angiography. Am J Ophthalmol. 1995; 120:642–51.
Article8. Nagaoka T, Kitaya N, Sugawara R. . Alteration of choroidal circulation in the foveal region in patients with type 2 diabetes. Br J Ophthalmol. 2004; 88:1060–3.
Article9. Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008; 146:496–500.
Article10. Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009; 147:811–5.
Article11. Fujiwara T, Imamura Y, Margolis R. . Enhanced depth imaging optical coherence tomography of the choroid in highly myopic eyes. Am J Ophthalmol. 2009; 148:445–50.
Article12. Imamura Y, Fujiwara T, Margolis R, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in central se-rous chorioretinopathy. Retina. 2009; 29:1469–73.
Article13. Spaide RF. Age-related choroidal atrophy. Am J Ophthalmol. 2009; 147:801–10.
Article14. Manjunath V, Taha M, Fujimoto JG, Duker JS. Choroidal thickness in normal eyes measured using Cirrus HD optical coherence tomography. Am J Ophthalmol. 2010; 150:325–9.e1.
Article15. Ikuno Y, Kawaguchi K, Nouchi T, Yasuno Y. Choroidal thickness in healthy Japanese subjects. Invest Ophthalmol Vis Sci. 2010; 51:2173–6.
Article16. Esmaeelpour M, Považay B, Hermann B. . Mapping choroidal and retinal thickness variation in type 2 diabetes using three-di-mensional 1060-nm optical coherence tomography. Invest Ophthalmol Vis Sci. 2011; 52:5311–6.
Article17. Regatieri CV, Branchini L, Carmody J. . Choroidal thickness in patients with diabetic retinopathy analyzed by spectral-domain optical coherence tomography. Retina. 2012; 32:563–8.
Article18. Hee MR, Puliafito CA, Duker JS. . Topography of diabetic macular edema with optical coherence tomography. Ophthalmology. 1998; 105:360–70.
Article19. Early Treatment Diabetic Retinopathy Study design and baseline patient characteristics ETDRS report number 7. Ophthalmology. 1991; 98:(5 Suppl). 741–56.20. Davis MD. Vitreous contraction in proliferative diabetic retinopathy. Arch Ophthalmol. 1965; 74:741–51.
Article21. Taylor E, Dobree JH. Proliferative diabetic retinopathy. Site and size of initial lesions. Br J Ophthalmol. 1970; 54:11–8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Choroidal Thickness in Patients with Diabetes by Spectral-domain Optical Coherence Tomography
- Change in Subfoveal Choroidal Thickness after Patterned Panretinal Photocoagulation in Patients with Diabetic Retinopathy
- Difference of GCIPL Thickness of Diabetes and Normal Eyes in Spectral Domain OCT
- Effects of Diabetic Retinopathy and Intravitreal Bevacizumab Injection on Choroidal Thickness in Diabetic Patients
- The Posterior Choroidal Profiles Measured by Spectral Domain Optical Coherence Tomography in Healthy Korean Children