Cancer Res Treat.
2015 Jul;47(3):518-526. 10.4143/crt.2013.241.
Improved Efficacy of a Dendritic Cell-Based Vaccine against a Murine Model of Colon Cancer: The Helper Protein Effect
- Affiliations
-
- 1Nanobiotechnology Research Center, Avicenna Research Institute, ACECR, Tehran, Iran. zarnani@avicenna.ac.ir
- 2Immunology Research Center, Iran University of Medical Sciences, Tehran, Iran.
- 3Department of Immunology, Iran University of Medical Sciences, Tehran, Iran.
- 4Independent Research Consultant, Toronto, Canada.
- KMID: 2148503
- DOI: http://doi.org/10.4143/crt.2013.241
Abstract
- PURPOSE
Targeted immunotherapy using dendritic cells (DCs) has been employed in numerous investigations aiming at combating neoplasms. We previously showed that copulsing of an antigen with a helper protein could considerably enhance antigen presenting capacity of ex vivo-generated DCs. In this study, we attempted to administer an effective treatment in a murine model of colon cancer with DCs pulsed with the mixture of a tumor-specific gp70-derived peptide (AH1) and a helper protein, ovalbumin (OVA).
MATERIALS AND METHODS
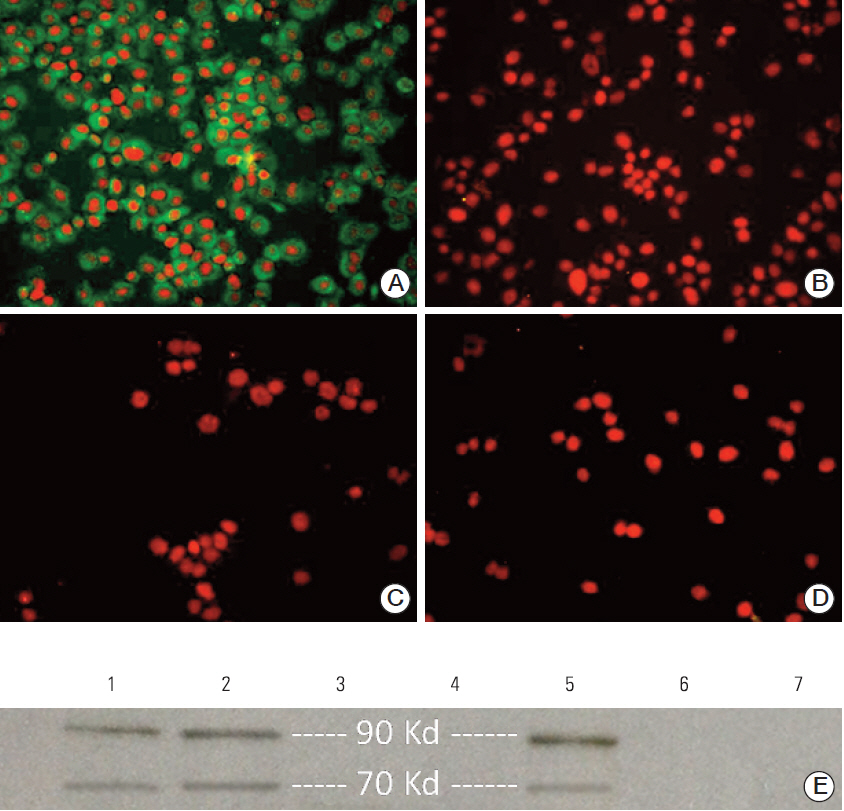
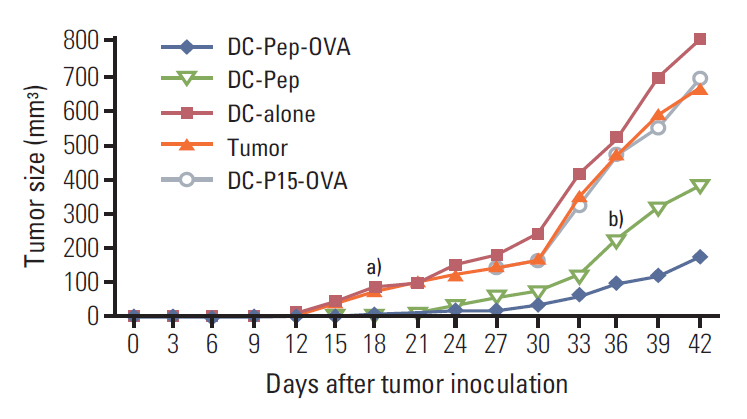
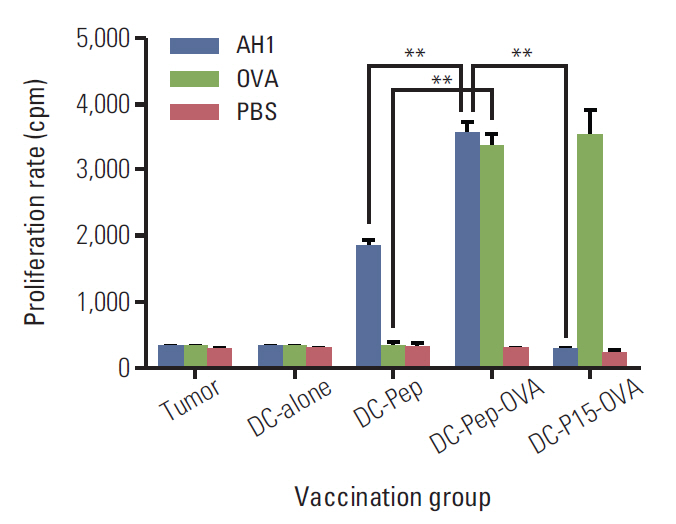
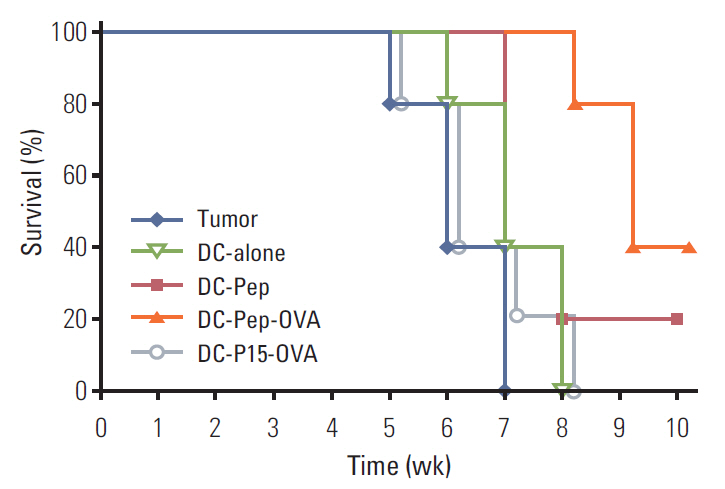
First, the presence of gp70 in CT26 tumor cells and tumor tissues was verified using immunofluorescence and Western blot analyses. Next, DCs were purified from normal mice, loaded ex vivowith AH1 and OVA (DC-Pep-OVA), and injected into tumor-bearing mice. Tumor volume, in vitro antigen (Ag)-specific proliferation of splenic cells, and survival rate were measured to determine the efficacy of DC-Pep-OVA. As the control groups, tumor-bearing mice were vaccinated with DC-Pep, unpulsed DC, and DCs loaded with a mixture of OVA and an irrelevant peptide (P15), or were not vaccinated at all.
RESULTS
DC-Pep-OVA showed superior efficacy over other groups, as indicated by smaller tumor volume, higher Ag-specific proliferation rate of splenic cells, and prolonged survival.
CONCLUSION
Overall, in the present study we showed for the first time that DCs copulsed with AH1 (tumor Ag) and OVA (helper molecule) could be considered as potentially robust weapons for use in future antitumor immunotherapies.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Combination Treatment of Stereotactic Body Radiation Therapy and Immature Dendritic Cell Vaccination for Augmentation of Local and Systemic Effects
Chul Won Choi, Min Ho Jeong, You-Soo Park, Cheol-Hun Son, Hong-Rae Lee, Eun-Kyoung Koh
Cancer Res Treat. 2019;51(2):464-473. doi: 10.4143/crt.2018.186.T Cells Modified with CD70 as an Alternative Cellular Vaccine for Antitumor Immunity
Sang-Eun Lee, A-Ri Shin, Hyun-Jung Sohn, Hyun-Il Cho, Tai-Gyu Kim
Cancer Res Treat. 2020;52(3):747-763. doi: 10.4143/crt.2019.721.
Reference
-
References
1. Voss CY, Albertini MR, Malter JS. Dendritic cell-based immunotherapy for cancer and relevant challenges for transfusion medicine. Transfus Med Rev. 2004; 18:189–202.
Article2. Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012; 12:265–77.
Article3. Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012; 12:269–81.
Article4. Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer. 2012; 12:278–87.
Article5. Aptsiauri N, Cabrera T, Garcia-Lora A, Garrido F. Cancer immune escape: implications for immunotherapy, Granada, Spain, October 3-5, 2011. Cancer Immunol Immunother. 2012; 61:739–45.
Article6. Torabi-Rahvar M, Bozorgmehr M, Jeddi-Tehrani M, Zarnani AH. Potentiation strategies of dendritic cell-based antitumor vaccines: combinational therapy takes the front seat. Drug Discov Today. 2011; 16:733–40.
Article7. Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012; 12:237–51.
Article8. Shimizu K, Thomas EK, Giedlin M, Mule JJ. Enhancement of tumor lysate- and peptide-pulsed dendritic cell-based vaccines by the addition of foreign helper protein. Cancer Res. 2001; 61:2618–24.9. Timmerman JM, Levy R. Linkage of foreign carrier protein to a self-tumor antigen enhances the immunogenicity of a pulsed dendritic cell vaccine. J Immunol. 2000; 164:4797–803.
Article10. Timmerman JM, Czerwinski DK, Davis TA, Hsu FJ, Benike C, Hao ZM, et al. Idiotype-pulsed dendritic cell vaccination for B-cell lymphoma: clinical and immune responses in 35 patients. Blood. 2002; 99:1517–26.
Article11. Millard AL, Ittelet D, Schooneman F, Bernard J. Dendritic cell KLH loading requirements for efficient CD4+ T-cell priming and help to peptide-specific cytotoxic T-cell response, in view of potential use in cancer vaccines. Vaccine. 2003; 21:869–76.
Article12. Shojaeian J, Jeddi-Tehrani M, Dokouhaki P, Mahmoudi AR, Ghods R, Bozorgmehr M, et al. Mutual helper effect in copulsing of dendritic cells with 2 antigens: a novel approach for improvement of dendritic-based vaccine efficacy against tumors and infectious diseases simultaneously. J Immunother. 2009; 32:325–32.13. Casares N, Lasarte JJ, de Cerio AL, Sarobe P, Ruiz M, Melero I, et al. Immunization with a tumor-associated CTL epitope plus a tumor-related or unrelated Th1 helper peptide elicits protective CTL immunity. Eur J Immunol. 2001; 31:1780–9.
Article14. Wierecky J, Muller MR, Wirths S, Halder-Oehler E, Dorfel D, Schmidt SM, et al. Immunologic and clinical responses after vaccinations with peptide-pulsed dendritic cells in metastatic renal cancer patients. Cancer Res. 2006; 66:5910–8.
Article15. Zarnani AH, Shahbazi M, Salek-Moghaddam A, Zareie M, Tavakoli M, Ghasemi J, et al. Vitamin D3 receptor is expressed in the endometrium of cycling mice throughout the estrous cycle. Fertil Steril. 2010; 93:2738–43.
Article16. Shojaeian S, Allameh A, Zarnani AH, Chamankhah M, Ghods R, Bayat AA, et al. Production and characterization of monoclonal antibodies against the extracellular domain of CA 125. Immunol Invest. 2010; 39:114–31.
Article17. Shahbazi M, Jeddi-Tehrani M, Zareie M, Salek-Moghaddam A, Akhondi MM, Bahmanpoor M, et al. Expression profiling of vitamin D receptor in placenta, decidua and ovary of pregnant mice. Placenta. 2011; 32:657–64.
Article18. Zarnani AH, Moazzeni SM, Shokri F, Salehnia M, Dokouhaki P, Shojaeian J, et al. The efficient isolation of murine splenic dendritic cells and their cytochemical features. Histochem Cell Biol. 2006; 126:275–82.
Article19. Zarnani AH, Moazzeni SM, Shokri F, Salehnia M, Dokouhaki P, Ghods R, et al. Microenvironment of the feto-maternal interface protects the semiallogenic fetus through its immunomodulatory activity on dendritic cells. Fertil Steril. 2008; 90:781–8.
Article20. Mimura Y, Mimura-Kimura Y, Doores K, Golgher D, Davis BG, Dwek RA, et al. Folding of an MHC class II-restricted tumor antigen controls its antigenicity via MHC-guided processing. Proc Natl Acad Sci U S A. 2007; 104:5983–8.
Article21. Steinman RM. Decisions about dendritic cells: past, present, and future. Annu Rev Immunol. 2012; 30:1–22.
Article22. Eriksson K, Sun JB, Nordstrom I, Fredriksson M, Lindblad M, Li BL, et al. Coupling of antigen to cholera toxin for dendritic cell vaccination promotes the induction of MHC class I-restricted cytotoxic T cells and the rejection of a cognate antigen-expressing model tumor. Eur J Immunol. 2004; 34:1272–81.
Article23. Eriksson K, Fredriksson M, Nordstrom I, Holmgren J. Cholera toxin and its B subunit promote dendritic cell vaccination with different influences on Th1 and Th2 development. Infect Immun. 2003; 71:1740–7.
Article24. George-Chandy A, Eriksson K, Lebens M, Nordstrom I, Schon E, Holmgren J. Cholera toxin B subunit as a carrier molecule promotes antigen presentation and increases CD40 and CD86 expression on antigen-presenting cells. Infect Immun. 2001; 69:5716–25.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Induction of 90K-specific Cytotoxic T Lymphocytes for Colon Cancer Immunotherapy
- Cancer Vaccines
- The Clinical Impact of the Dendritic Cell-based Cancer Vaccine: the Role in the Inflammatory Tumor Micro-environment
- Enhanced anti-tumor immunity of vaccine combined with anti-PD-1 antibody in a murine bladder cancer model
- Specific Immunotherapy Using Autologous Tumor Vaccine Treats Mutine Metastatic Hepatic Cancer