Immune Netw.
2010 Dec;10(6):206-211. 10.4110/in.2010.10.6.206.
Induction of 90K-specific Cytotoxic T Lymphocytes for Colon Cancer Immunotherapy
- Affiliations
-
- 1Department of Oncology, Chonnam National University Hwasun Hospital, Hwasun 519-809, Korea. ijchung@chonnam.ac.kr
- KMID: 2150676
- DOI: http://doi.org/10.4110/in.2010.10.6.206
Abstract
- BACKGROUND
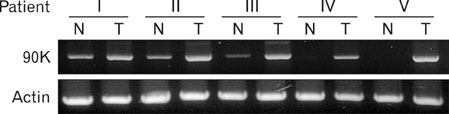
Dendritic cell (DC)-based tumor vaccine is an attractive modality for the treatment of colon cancer because it has been recurred and produced few side effects in patients. Secretory glycoprotein 90K has been found at elevated level in various cancer tissues and sera. We investigated to establish a more effective DC vaccine for the treatment of colon cancer in which the levels of 90K are elevated.
METHODS
We obtained the concentrated 90K from 293T cells stably expressing 90K. DCs were cultured from peripheral blood monocytes, and a DC vaccine pulsed with tumor lysate was compared with a DC vaccine pulsed with 90K. We measured the functional activity for CTLs by using IFN-gamma-enzyme linked immunoabsorbent spot (ELISPOT) assay.
RESULTS
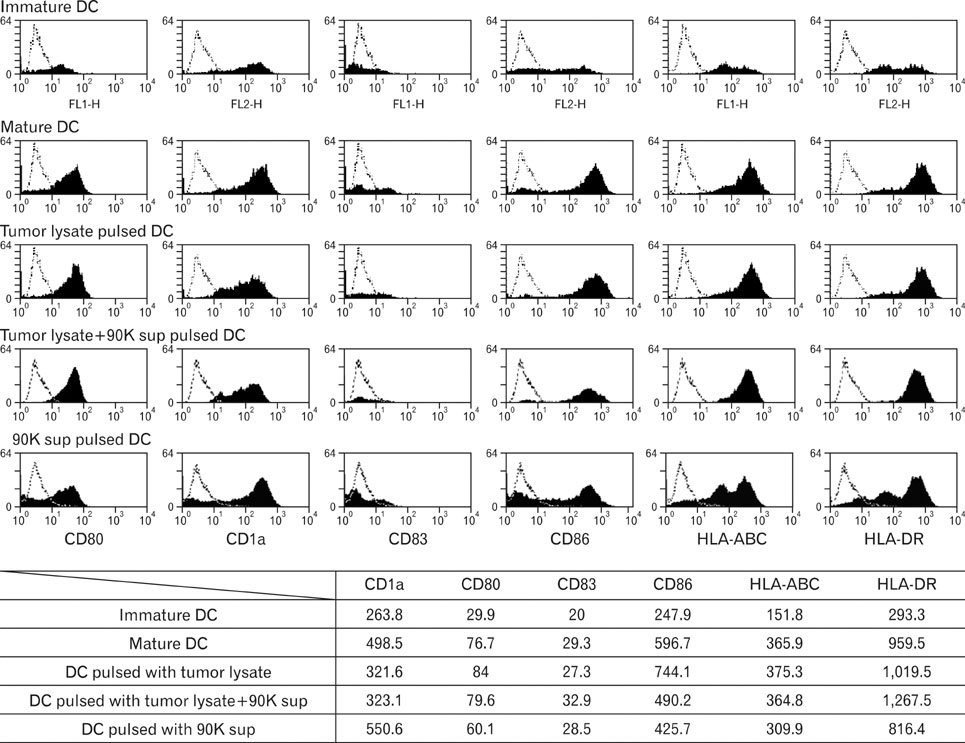
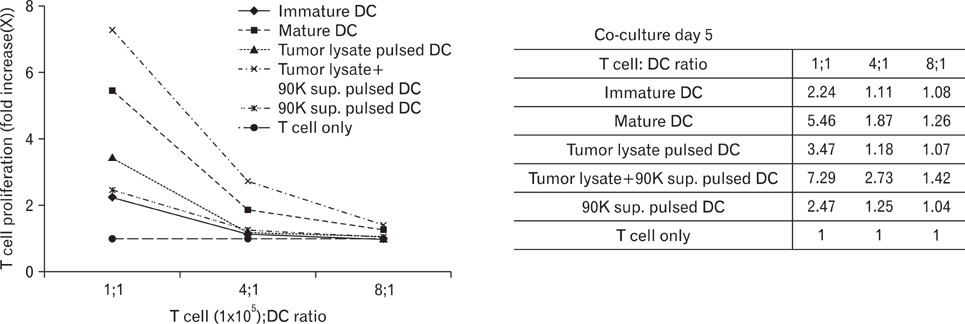
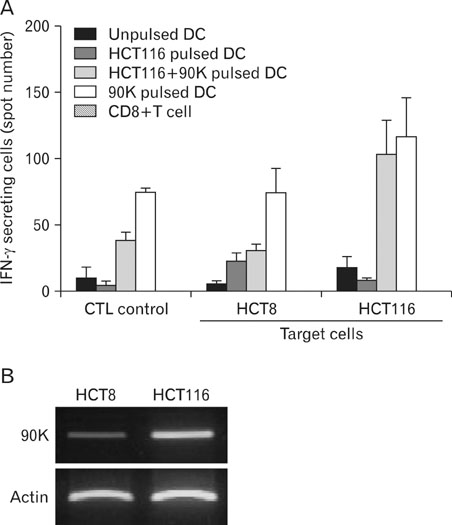
DCs pulsed with tumor lysate+90K exhibited the enhanced T cell stimulation, polarization of naive T cell toward Th1. The CTLs generated by DCs pulsed with 90K efficiently lysed HCT116 cells. The results indicate that 90K-speicifc-CTLs can recognize 90K proteins naturally presented by colon cancer cells.
CONCLUSION
Our study suggests that 90K-specific CTLs generated by 90K-pulsed DCs could be useful effector cells for immunotherapy in colon cancer.
Keyword
MeSH Terms
Figure
Reference
-
1. Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998. 392:245–252.
Article2. Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000. 18:767–811.
Article3. Steinman RM, Dhodapkar M. Active immunization against cancer with dendritic cells: the near future. Int J Cancer. 2001. 94:459–473.
Article4. Zitvogel L, Mayordomo JI, Tjandrawan T, DeLeo AB, Clarke MR, Lotze MT, Storkus WJ. Therapy of murine tumors with tumor peptide-pulsed dendritic cells: dependence on T cells, B7 costimulation, and T helper cell 1-associated cytokines. J Exp Med. 1996. 183:87–97.
Article5. Kaplan JM, Yu Q, Piraino ST, Pennington SE, Shankara S, Woodworth LA, Roberts BL. Induction of antitumor immunity with dendritic cells transduced with adenovirus vector-encoding endogenous tumor-associated antigens. J Immunol. 1999. 163:699–707.6. Iacobelli S, Arnò E, D'Orazio A, Coletti G. Detection of antigens recognized by a novel monoclonal antibody in tissue and serum from patients with breast cancer. Cancer Res. 1986. 46:3005–3010.7. Rosenberg I, Cherayil BJ, Isselbacher KJ, Pillai S. Mac-2-binding glycoproteins. Putative ligands for a cytosolic beta-galactoside lectin. J Biol Chem. 1991. 266:18731–18736.
Article8. Koths K, Taylor E, Halenbeck R, Casipit C, Wang A. Cloning and characterization of a human Mac-2-binding protein, a new member of the superfamily defined by the macrophage scavenger receptor cysteine-rich domain. J Biol Chem. 1993. 268:14245–14249.
Article9. Ullrich A, Sures I, D'Egidio M, Jallal B, Powell TJ, Herbst R, Dreps A, Azam M, Rubinstein M, Natoli C, et al. The secreted tumor-associated antigen 90K is a potent immune stimulator. J Biol Chem. 1994. 269:18401–18407.
Article10. Iacovazzi PA, Trisolini A, Barletta D, Elba S, Manghisi OG, Correale M. Serum 90K/MAC-2BP glycoprotein in patients with liver cirrhosis and hepatocellular carcinoma: a comparison with alpha-fetoprotein. Clin Chem Lab Med. 2001. 39:961–965.11. Fusco O, Querzoli P, Nenci I, Natoli C, Brakebush C, Ullrich A, Iacobelli S. 90K (MAC-2 BP) gene expression in breast cancer and evidence for the production of 90K by peripheral-blood mononuclear cells. Int J Cancer. 1998. 79:23–26.
Article12. Park YP, Choi SC, Kim JH, Song EY, Kim JW, Yoon DY, Yeom YI, Lim JS, Kim JW, Paik SG, Lee HG. Up-regulation of Mac-2 binding protein by hTERT in gastric cancer. Int J Cancer. 2007. 120:813–820.
Article13. Ozaki Y, Kontani K, Hanaoka J, Chano T, Teramoto K, Tezuka N, Sawai S, Fujino S, Yoshiki T, Okabe H, Ohkubo I. Expression and immunogenicity of a tumor-associated antigen, 90K/Mac-2 binding protein, in lung carcinoma. Cancer. 2002. 95:1954–1962.
Article14. Becker R, Lenter MC, Vollkommer T, Boos AM, Pfaff D, Augustin HG, Christian S. Tumor stroma marker endosialin (Tem1) is a binding partner of metastasis-related protein Mac-2 BP/90K. FASEB J. 2008. 22:3059–3067.
Article15. Mayordomo JI, Zorina T, Storkus WJ, Zitvogel L, Celluzzi C, Falo LD, Melief CJ, Ildstad ST, Kast WM, Deleo AB, et al. Bone marrow-derived dendritic cells pulsed with synthetic tumour peptides elicit protective and therapeutic antitumour immunity. Nat Med. 1995. 1:1297–1302.
Article16. Porgador A, Gillboa E. Bone marrow-generated dendritic cells pulsed with a class I-restricted peptide are potent inducers of cytotoxic T lymphocytes. J Exp Med. 1995. 182:255–260.
Article17. Boczkowski D, Nair SK, Snyder D, Gilboa E. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J Exp Med. 1996. 184:465–472.
Article18. Boczkowski D, Nair SK, Snyder D, Gilboa E. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J Exp Med. 1996. 184:465–472.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Adoptive Immunotherapy for Cytomegalovirus (CMV) Disease in Immunocompromised Patients
- Systemic Therapy for Advanced and Metastatic Colon Cancer
- Adoptive Transfer of Colon Cancer Derived Peptide-specific CD8+ T Cells in HHD Mice
- Anti-tumor Cytotoxicity of Allogeneic Neuroblastoma Tumor Antigen-loaded Dendiritic Cells
- Recent Updates in Cancer Immunotherapy