Anat Cell Biol.
2012 Dec;45(4):229-240. 10.5115/acb.2012.45.4.229.
Preliminary morphological and biochemical changes in rat liver following postnatal exposure to sodium arsenite
- Affiliations
-
- 1Department of Anatomy, All India Institute of Medical Sciences, New Delhi, India. dharpushpa@hotmail.com
- KMID: 2133909
- DOI: http://doi.org/10.5115/acb.2012.45.4.229
Abstract
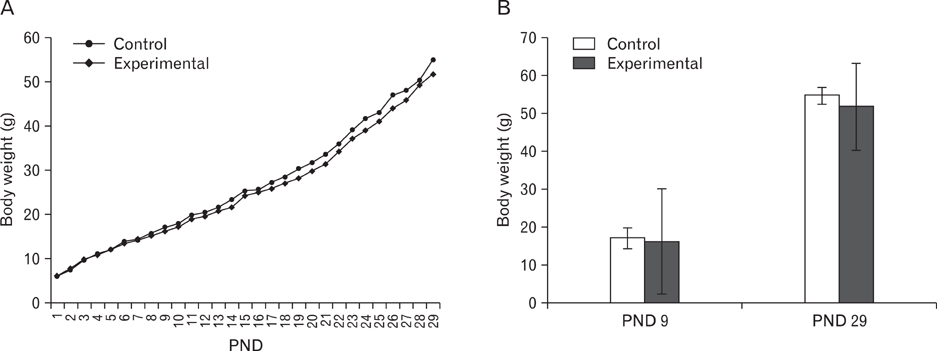
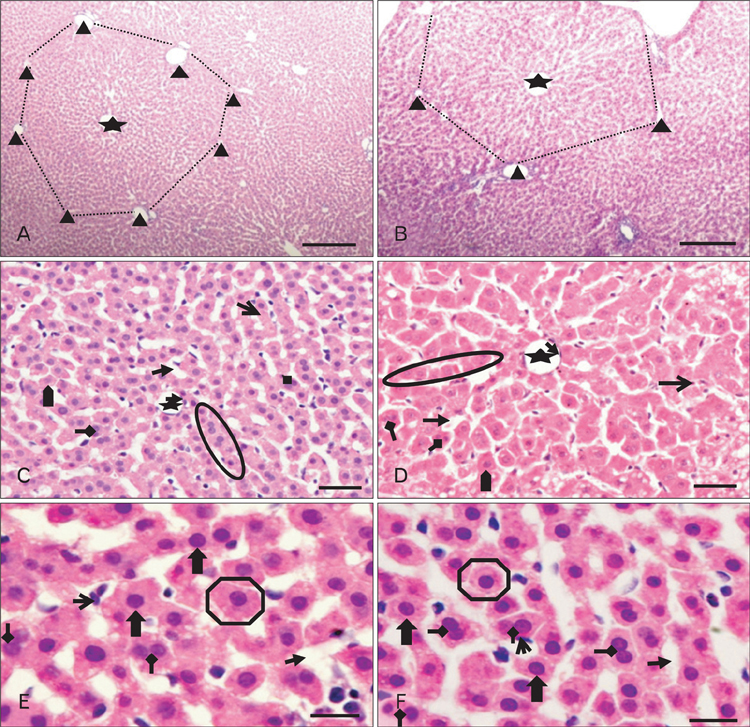
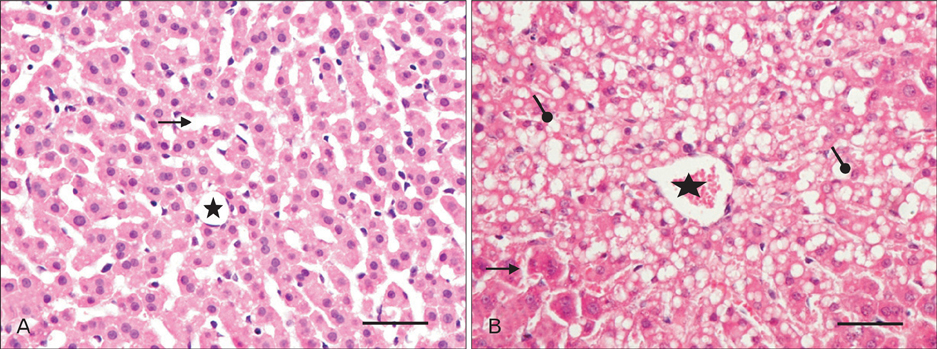
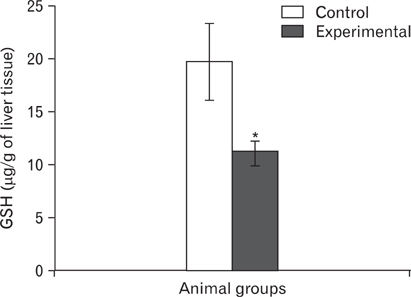
- The effects of sodium arsenite exposure on the hepatic maturation period of cellular and functional reorganization in developing rat livers were evaluated. Animals received intraperitoneal injections of sodium arsenite (1.5 mg/kg body weight) or distilled water on days 9 to 28 after birth. On day 29, the animals were sacrificed either by cervical dislocation or by perfusion fixation. The perfusion fixed liver tissue was processed for paraffin embedding, sectioning and hematoxylin and eosin staining. The fresh liver tissue was processed for cryo-sectioning followed by Sudan Black B staining and for biochemical estimation of reduced glutathione. Microscopic observation revealed comparable preserved hepatic lobular patterns and distributions of uninucleate and binucleate hepatocytes in the control and the experimental groups. The mean nuclear area and diameter of the hepatocytes was increased in the experimental group. Lipid droplet distribution pattern in Sudan Black B stained sections revealed higher staining intensity towards the centrilobular area in both groups. Semiquantitative estimation of staining intensity showed lower mean gray values in zone 3 than in zones 2 and 1 (suggestive of the setting in of the adult pattern) in both groups. The reduced glutathione levels in the liver tissue and the altered nuclear size of the hepatocytes in the experimental group suggested the impairment of morphological and biochemical processes induced by arsenic exposure during the postnatal period.
Keyword
MeSH Terms
-
Adult
Animals
Arsenic
Arsenites
Azo Compounds
Biochemical Processes
Dislocations
Eosine Yellowish-(YS)
Glutathione
Hematoxylin
Hepatocytes
Humans
Injections, Intraperitoneal
Liver
Paraffin Embedding
Parturition
Perfusion
Rats
Rats, Wistar
Sodium
Sodium Compounds
Sudan
Water
Arsenic
Arsenites
Azo Compounds
Eosine Yellowish-(YS)
Glutathione
Hematoxylin
Sodium
Sodium Compounds
Water
Figure
Reference
-
1. Garelick H, Jones H, Dybowska A, Valsami-Jones E. Arsenic pollution sources. Rev Environ Contam Toxicol. 2008. 197:17–60.2. Hardman JG, Limbird LE, Gilman AG. Goodman & Gilman's: the pharmacological basis of therapeutics. 2001. 10th ed. New York: McGraw-Hill;1892–1965.3. Agency for Toxic Substances and Disease Registry. 2007. Toxicological profile for arsenic. Draft for public comment [Internet]. 2007. cited 2012 Nov 1. Atlanta: Agency for Toxic Substances and Disease Registry;Available from: http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=22&tid=3.4. Sato EF, Nakagawa E, Hiramoto K, Yamamasu S, Moriyama-Shimamoto I, Inoue M. Oxidative stress promotes the regression of fetal liver hemopoiesis. Biochemistry (Mosc). 2004. 69:18–22.5. Alexander B, Guzail MA, Foster CS. Morphological changes during hepatocellular maturity in neonatal rats. Anat Rec. 1997. 248:104–109.6. Liu J, Xie Y, Ward JM, Diwan BA, Waalkes MP. Toxicogenomic analysis of aberrant gene expression in liver tumors and nontumorous livers of adult mice exposed in utero to inorganic arsenic. Toxicol Sci. 2004. 77:249–257.7. Ledda-Columbano GM, Coni P, Faa G, Manenti G, Columbano A. Rapid induction of apoptosis in rat liver by cycloheximide. Am J Pathol. 1992. 140:545–549.8. Sell S, Salman J. Light- and electron-microscopic autoradiographic analysis of proliferating cells during the early stages of chemical hepatocarcinogenesis in the rat induced by feeding N-2-fluorenylacetamide in a choline-deficient diet. Am J Pathol. 1984. 114:287–300.9. Farber E. Similarities in the sequence of early histological changes induced in the liver of the rat by ethionine, 2-acetylamino-fluorene, and 3'-methyl-4-dimethylaminoazobenzene. Cancer Res. 1956. 16:142–148.10. White EG. Some observations on the liver of the pig: the hepatic lobule and liver cell during post-natal growth. J Anat. 1939. 73(Pt 3):365–386.1.11. Asada M, Kanamura S. Postnatal changes in the distribution of lipid droplets within the liver lobule of the mouse. J Anat. 1979. 129(Pt 2):423–426.12. Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta. 1979. 582:67–78.13. Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959. 82:70–77.14. Bancroft JD, Gamble M. Theory and practice of histological techniques. 2002. 5th ed. Edinburgh: Churchill Livingstone;206–256.15. Carson FL. Histotechnology: a self instructional text. 1990. Chicago: ASCP Press;161–162.16. Bayliss High OB. The histochemical versatility of Sudan Black B. Acta Histochem Suppl. 1981. 24:247–255.17. Shen LJ, Zhang ZJ, Ou YM, Zhang HX, Huang R, He Y, Wang MJ, Xu GS. Computed morphometric analysis and expression of alpha fetoprotein in hepatocellular carcinoma and its related lesion. World J Gastroenterol. 2000. 6:415–416.18. Bjugn R. The use of the optical disector to estimate the number of neurons, glial and endothelial cells in the spinal cord of the mouse: with a comparative note on the rat spinal cord. Brain Res. 1993. 627:25–33.19. Ross MH, Romrell LJ, Kaye GI. Histology: a text and atlas. 1995. 3rd ed. Philadelphia: Williams & Wilkins;498–500.20. Zubair A, Jamal S, Mubarik A. Morphometric analysis of hepatic steatosis in chronic hepatitis C infection. Saudi J Gastroenterol. 2009. 15:11–14.21. Dhar P, Somesh M, Kaushal P, Sehgal R, Mehra RD. Effects of arsenic exposure during early postnatal period on Leydig cells of rat testis. Toxicol Environ Chem. 2010. 92:1157–1168.22. Dhar P, Mohari N, Mehra RD. Preliminary morphological and morphometric study of rat cerebellum following sodium arsenite exposure during rapid brain growth (RBG) period. Toxicology. 2007. 234:10–20.23. Rodríguez VM, Carrizales L, Mendoza MS, Fajardo OR, Giordano M. Effects of sodium arsenite exposure on development and behavior in the rat. Neurotoxicol Teratol. 2002. 24:743–750.24. Jana K, Jana S, Samanta PK. Effects of chronic exposure to sodium arsenite on hypothalamo-pituitary-testicular activities in adult rats: possible an estrogenic mode of action. Reprod Biol Endocrinol. 2006. 4:9.25. Pant N, Murthy RC, Srivastava SP. Male reproductive toxicity of sodium arsenite in mice. Hum Exp Toxicol. 2004. 23:399–403.26. Sarkar M, Chaudhuri GR, Chattopadhyay A, Biswas NM. Effect of sodium arsenite on spermatogenesis, plasma gonadotrophins and testosterone in rats. Asian J Androl. 2003. 5:27–31.27. Holson JF, Stump DG, Ulrich CE, Farr CH. Absence of prenatal developmental toxicity from inhaled arsenic trioxide in rats. Toxicol Sci. 1999. 51:87–97.28. Datta S, Saha DR, Ghosh D, Majumdar T, Bhattacharya S, Mazumder S. Sub-lethal concentration of arsenic interferes with the proliferation of hepatocytes and induces in vivo apoptosis in Clarias batrachus L. Comp Biochem Physiol C Toxicol Pharmacol. 2007. 145:339–349.29. Braet F, Vanbesien J, De Zanger R, Wisse E. Ageing of the liver sieve and pseudocapillarisation. Lancet. 2002. 360:1171–1172.30. Straub AC, Stolz DB, Ross MA, Hernández-Zavala A, Soucy NV, Klei LR, Barchowsky A. Arsenic stimulates sinusoidal endothelial cell capillarization and vessel remodeling in mouse liver. Hepatology. 2007. 45:205–212.31. Straub AC, Stolz DB, Vin H, Ross MA, Soucy NV, Klei LR, Barchowsky A. Low level arsenic promotes progressive inflammatory angiogenesis and liver blood vessel remodeling in mice. Toxicol Appl Pharmacol. 2007. 222:327–336.32. Popper H, Thomas LB, Telles NC, Falk H, Selikoff IJ. Development of hepatic angiosarcoma in man induced by vinyl chloride, thorotrast, and arsenic. Comparison with cases of unknown etiology. Am J Pathol. 1978. 92:349–376.33. Sasaki K, Matsumura G. Haemopoietic cells of yolk sac and liver in the mouse embryo: a light and electron microscopical study. J Anat. 1986. 148:87–97.34. Chen ML, Gerber MA, Thung SN, Thornton JC, Chung WK. Morphometric study of hepatocytes containing hepatitis B surface antigen. Am J Pathol. 1984. 114:217–221.35. McKellar M. The postnatal growth and mitotic activity of the liver of the albino rat. Am J Anat. 1949. 85:263–307.36. Plenk H. Uber Anderungen der Zellgrosse im Zusammenhang mit dem Korperwachstum der Tiere. Arb Zool Inst Wien. 1911. 19:247–288.37. Sorensen EM, Ramirez-Mitchell R, Pradzynski A, Bayer TL, Wenz LL. Stereological analyses of hepatocyte changes parallel arsenic accumulation in the livers of green sunfish. J Environ Pathol Toxicol Oncol. 1985. 6:195–210.38. Bolender RP, Weibel ER. A morphometric study of the removal of phenobarbital-induced membranes from hepatocytes after cessation of threatment. J Cell Biol. 1973. 56:746–761.39. Barka T, Popper H. Liver enlargement and drug toxicity. Medicine (Baltimore). 1967. 46:103–117.40. Kunz W, Schaude G, Schmid W, Siess M. Stimulation of liver growth by drugs. Proceedings of the European Society for the Study of Drug Toxicity. 1966. 01. Vol. VII (International Congress Series No. 115):Rome. Amsterdam: Excerpta Medica Foundation;113–153.41. Blendis LM, Orrego H, Crossley IR, Blake JE, Medline A, Isreal Y. The role of hepatocyte enlargement in hepatic pressure in cirrhotic and noncirrhotic alcoholic liver disease. Hepatology. 1982. 2:539–546.42. Israel Y, Orrego H, Colman JC, Britton RS. Alcohol-induced hepatomegaly: pathogenesis and role in the production of portal hypertension. Fed Proc. 1982. 41:2472–2477.43. Watanabe T, Tanaka Y. Age-related alterations in the size of human hepatocytes. A study of mononuclear and binucleate cells. Virchows Arch B Cell Pathol Incl Mol Pathol. 1982. 39:9–20.44. Orrego H, Blendis LM, Crossley IR, Medline A, Macdonald A, Ritchie S, Israel Y. Correlation of intrahepatic pressure with collagen in the Disse space and hepatomegaly in humans and in the rat. Gastroenterology. 1981. 80:546–556.45. Ranek L, Keiding N, Jensen ST. A morphometric study of normal human liver cell nuclei. Acta Pathol Microbiol Scand A. 1975. 83:467–476.46. Park S, Jung Y. Combined actions of Na/K-ATPase, NCX1 and glutamate dependent NMDA receptors in ischemic rat brain penumbra. Anat Cell Biol. 2010. 43:201–210.47. Miyake H, Kadoya A, Ohyashiki T. Increase in molecular rigidity of the protein conformation of brain Na+-K+-ATPase by modification with 4-hydroxy-2-nonenal. Biol Pharm Bull. 2003. 26:1652–1656.48. Valkonen S, Savolainen H, Järvisalo J. Arsenic distribution and neurochemical effects in peroral sodium arsenite exposure of rats. Bull Environ Contam Toxicol. 1983. 30:303–308.49. Forbush B 3rd. Rapid 86Rb release from an occluded state of the Na,K-pump reflects the rate of dephosphorylation or dearsenylation. J Biol Chem. 1988. 263:7961–7969.50. Santra A, Maiti A, Das S, Lahiri S, Charkaborty SK, Mazumder DN. Hepatic damage caused by chronic arsenic toxicity in experimental animals. J Toxicol Clin Toxicol. 2000. 38:395–405.51. Ramos O, Carrizales L, Yáñez L, Mejía J, Batres L, Ortíz D, Díaz-Barriga F. Arsenic increased lipid peroxidation in rat tissues by a mechanism independent of glutathione levels. Environ Health Perspect. 1995. 103:Suppl 1. 85–88.52. Serhan MF, Kreydiyyeh SI. Insulin down-regulates the Na+/K+ ATPase in enterocytes but increases intestinal glucose absorption. Gen Comp Endocrinol. 2010. 167:228–233.53. Rosta K, Tulassay E, Enzsoly A, Ronai K, Szantho A, Pandics T, Fekete A, Mandl P, Ver A. Insulin induced translocation of Na+/K+-ATPase is decreased in the heart of streptozotocin diabetic rats. Acta Pharmacol Sin. 2009. 30:1616–1624.54. Lim DK, Kim HS. Opposite modulation of glutamate uptake by nicotine in cultured astrocytes with/without cAMP treatment. Eur J Pharmacol. 2003. 476:179–184.55. Suketa Y. Fundamental and applied studies on transport and metabolism of electrolytes and glucose: aim to contact with molecular biology. Yakugaku Zasshi. 2002. 122:507–525.56. Harold DE, Walz W. Metabolic inhibition and electrical properties of type-1-like cortical astrocytes. Neuroscience. 1992. 47:203–211.57. Guidotti JE, Brégerie O, Robert A, Debey P, Brechot C, Desdouets C. Liver cell polyploidization: a pivotal role for binuclear hepatocytes. J Biol Chem. 2003. 278:19095–19101.58. Pfuhl W. Mollendorf WV, editor. Die Leber. Handbuch der Mikroskopischen Anatomie des Menschen. 1932. Vol. 5. Berlin: Springer;235–425.59. Altunkaynak BZ, Ozbek E. Overweight and structural alterations of the liver in female rats fed a high-fat diet: a stereological and histological study. Turk J Gastroenterol. 2009. 20:93–103.60. Cutts JH, Leeson CR, Krause WJ. The postnatal development of the liver in a marsupial, Didelphis virginiana. 1. Light microscopy. J Anat. 1973. 115(Pt 3):327–346.61. Leeson CR, Cutts JH. The postnatal development of the rabbit liver. Biol Neonate. 1972. 20:404–413.62. Deane HW. A cytological study of storage and secretion in the developing liver of the mouse. Anat Rec. 1944. 88:161–173.63. McGovern BH, Ditelberg JS, Taylor LE, Gandhi RT, Christopoulos KA, Chapman S, Schwartzapfel B, Rindler E, Fiorino AM, Zaman MT, Sax PE, Graeme-Cook F, Hibberd PL. Hepatic steatosis is associated with fibrosis, nucleoside analogue use, and hepatitis C virus genotype 3 infection in HIV-seropositive patients. Clin Infect Dis. 2006. 43:365–372.64. Flora SJ, Pant SC, Malhotra PR, Kannan GM. Biochemical and histopathological changes in arsenic-intoxicated rats coexposed to ethanol. Alcohol. 1997. 14:563–568.65. Vahter M, Marafante E. Effects of low dietary intake of methionine, choline or proteins on the biotransformation of arsenite in the rabbit. Toxicol Lett. 1987. 37:41–46.66. Palekar AG, Tate SS, Meister A. Decrease in glutathione levels of kidney and liver after injection of methionine sulfoximine into rats. Biochem Biophys Res Commun. 1975. 62:651–657.67. Flora SJ, Chouhan S, Kannan GM, Mittal M, Swarnkar H. Combined administration of taurine and monoisoamyl DMSA protects arsenic induced oxidative injury in rats. Oxid Med Cell Longev. 2008. 1:39–45.68. Saha B. Effect of ascorbic acid on reduced glutathione level in arsenic-loaded isolated liver tissues of rat. Bangladesh J Pharmacol. 2006. 1:68–71.69. Vahter M. What are the chemical forms of arsenic in urine, and what can they tell us about exposure? Clin Chem. 1994. 40:679–680.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Acute Toxicity of Arsenic in Rats and Mice
- A Quantitative Morphologic Analysis of the Rat's Liver in the Postnatal Period
- Experimental Liver Disease Models of Rats: Morphological Characteristics
- Morphometric Study of Synapses in the Rat Cerebellar Cortex in Their Early Postnatal Periods
- Sodium and Water Retention in Liver Cirrhosis: Causes and Consequences