J Korean Med Sci.
2014 Aug;29(8):1042-1053. 10.3346/jkms.2014.29.8.1042.
Withaferin A-Caused Production of Intracellular Reactive Oxygen Species Modulates Apoptosis via PI3K/Akt and JNKinase in Rabbit Articular Chondrocytes
- Affiliations
-
- 1Department of Biological Sciences, Kongju National University, Gongju, Korea. ksj85@kongju.ac.kr
- KMID: 2129597
- DOI: http://doi.org/10.3346/jkms.2014.29.8.1042
Abstract
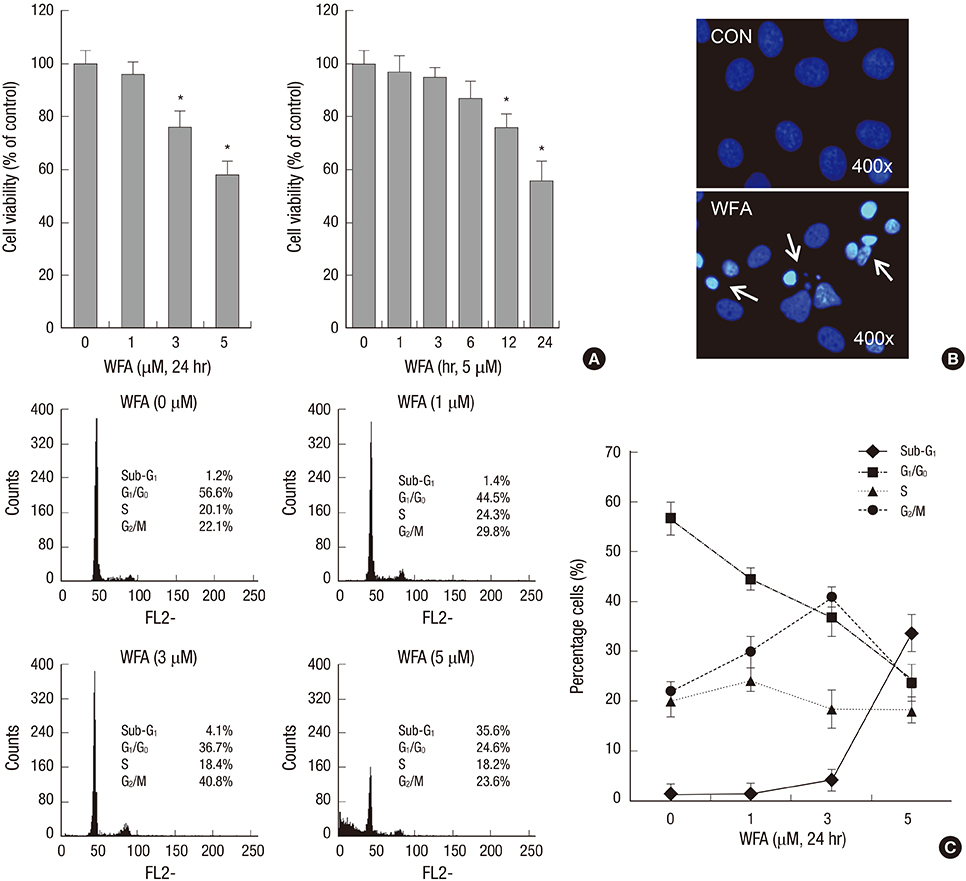
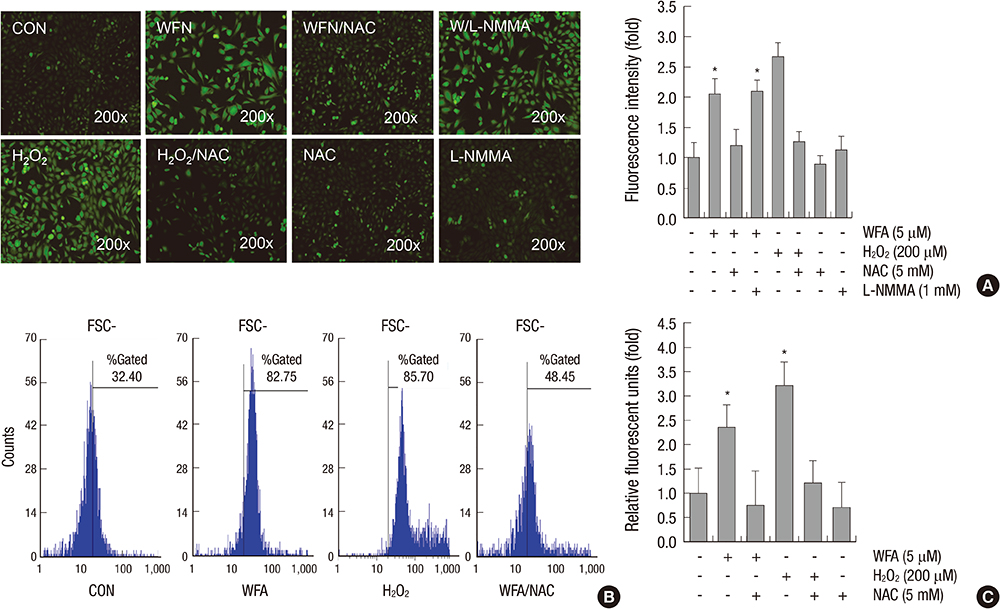
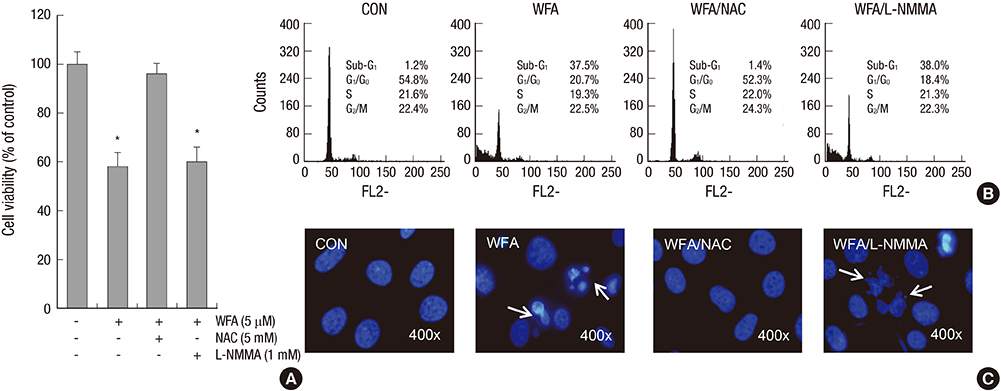
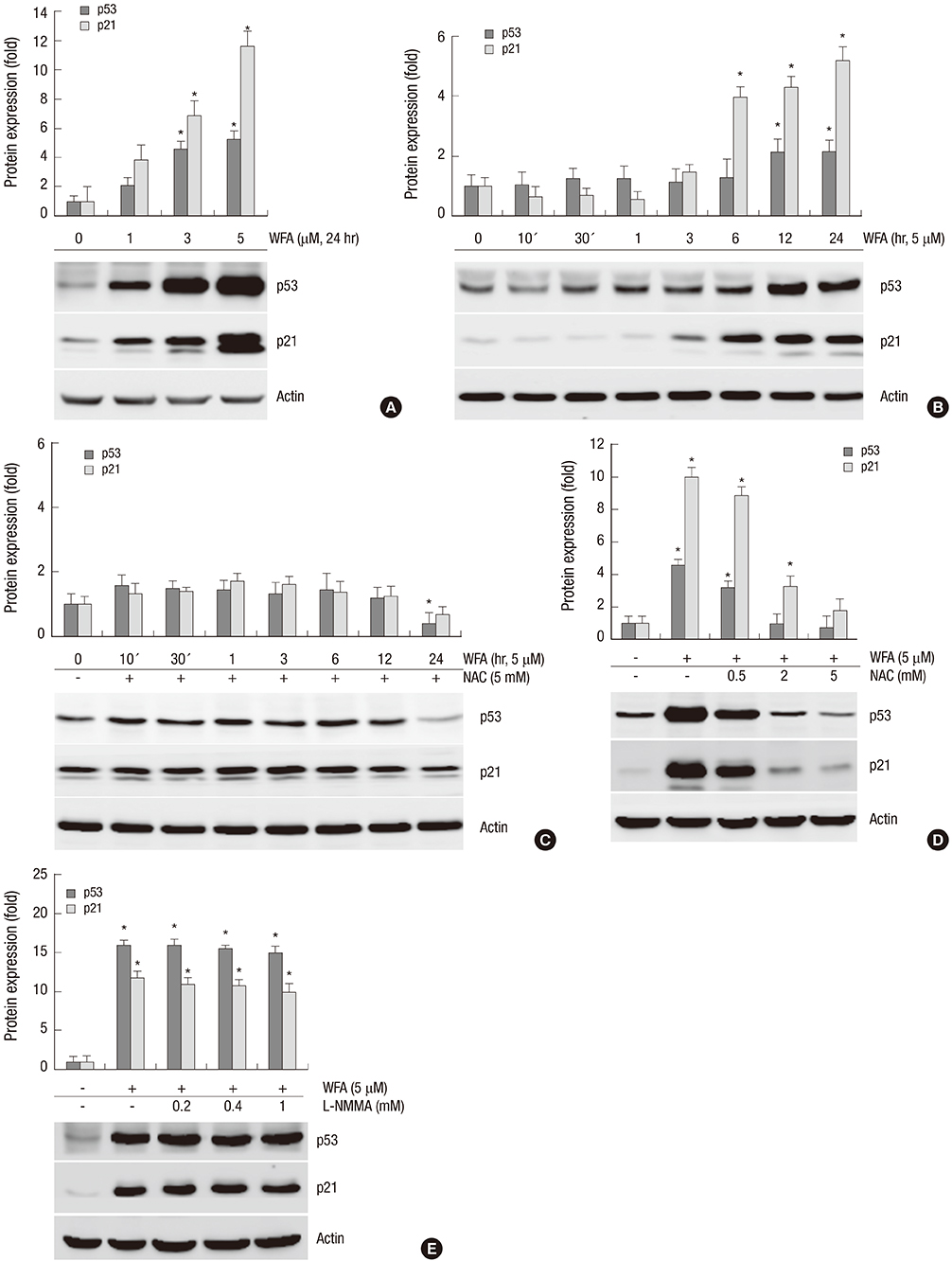
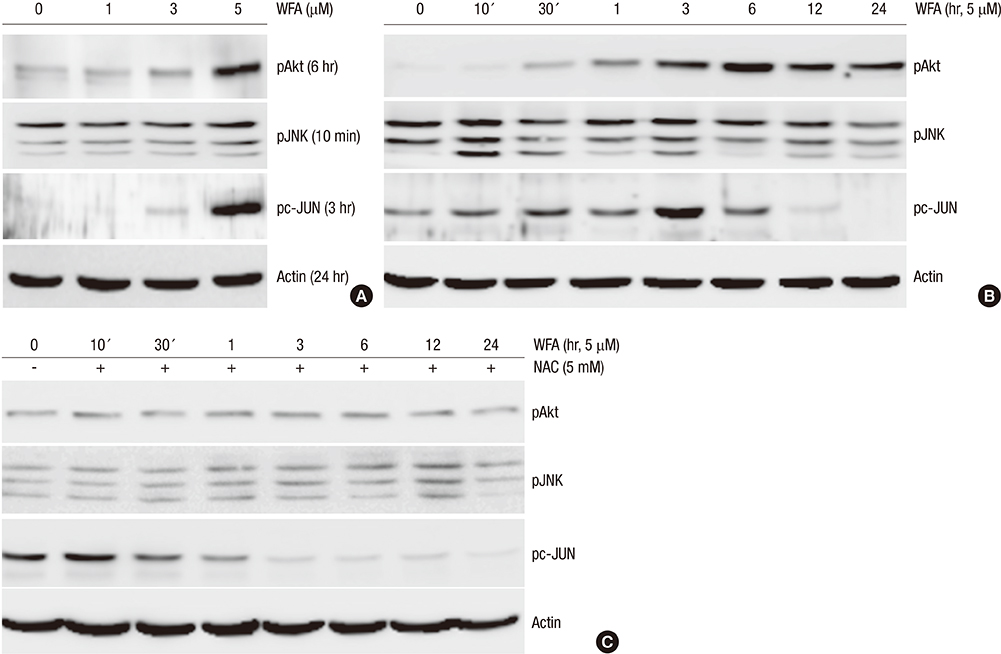
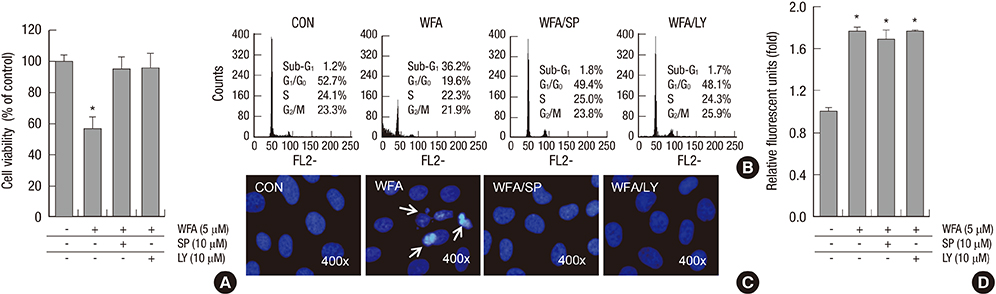
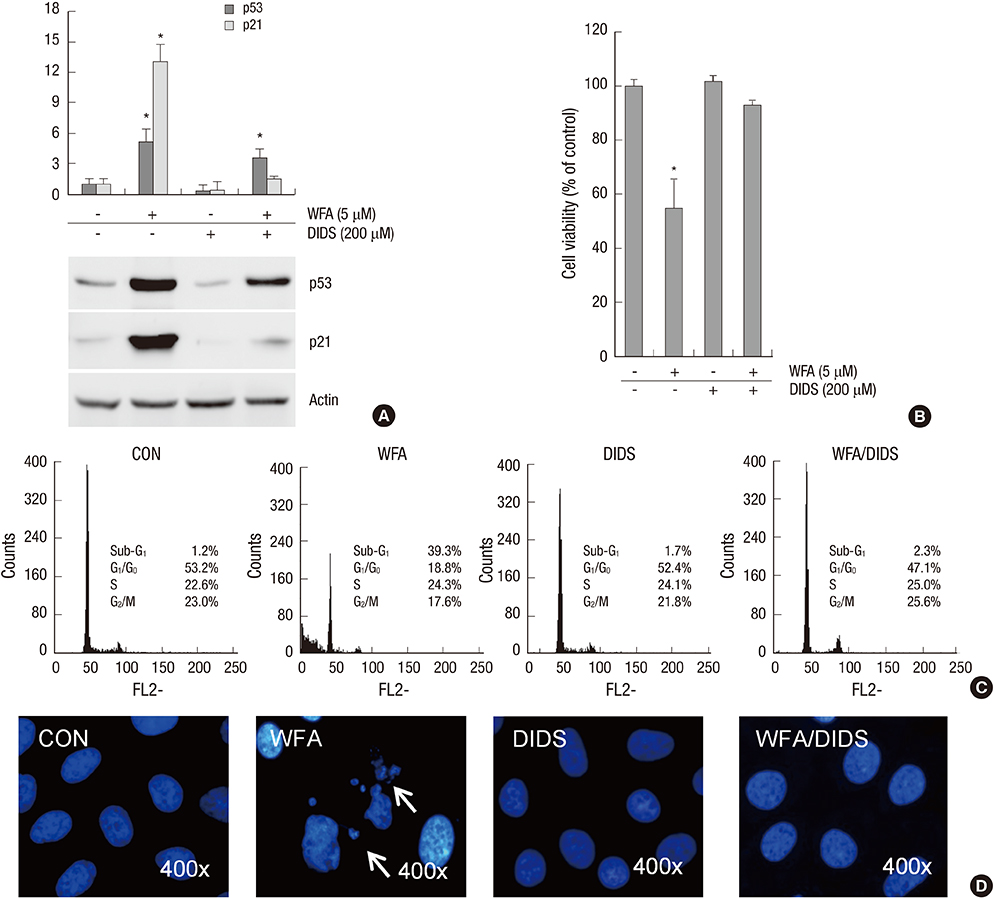
- Withaferin A (WFA) is known as a constituent of Ayurvedic medicinal plant, Withania somnifera, and has been used for thousands of years. Although WFA has been used for the treatment of osteoarthritis (OA) and has a wide range of biochemical and pharmacologic activities, there are no findings suggesting its properties on chondrocytes or cartilage. The aim of the present study is to investigate the effects of WFA on apoptosis with focus on generation of intracellular reactive oxygen species (ROS). Here we showed that WFA significantly increased the generation of intracellular ROS in a dose-dependent manner. We also determined that WFA markedly leads to apoptosis as evidenced by accumulation of p53 by Western blot analysis. N-Acetyl-L-Cystein (NAC), an antioxidant, prevented WFA-caused expression of p53 and inhibited apoptosis of chondrocytes. We also found that WFA causes the activation of PI3K/Akt and JNKinase. Inhibition of PI3K/Akt and JNKinase with LY294002 (LY)/triciribine (TB) or SP600125 (SP) in WFA-treated cells reduced accumulation of p53 and inhibited fragmented DNA. Our findings suggested that apoptosis caused by WFA-induced intracellular ROS generation is regulated through PI3K/Akt and JNKinase in rabbit articular chondrocytes.
Keyword
MeSH Terms
-
Animals
Anti-Inflammatory Agents/administration & dosage
Apoptosis/drug effects/physiology
Cartilage, Articular/cytology/drug effects/*metabolism
Cells, Cultured
Chondrocytes/drug effects/*metabolism
Dose-Response Relationship, Drug
MAP Kinase Kinase 4/*metabolism
Phosphatidylinositol 3-Kinases/*metabolism
Proto-Oncogene Proteins c-akt/metabolism
Rabbits
Reactive Oxygen Species/*metabolism
Withanolides/*administration & dosage
Anti-Inflammatory Agents
MAP Kinase Kinase 4
Proto-Oncogene Proteins c-akt
Phosphatidylinositol 3-Kinases
Reactive Oxygen Species
Withanolides
Figure
Reference
-
1. Thomas CM, Fuller CJ, Whittles CE, Sharif M. Chondrocyte death by apoptosis is associated with the initiation and severity of articular cartilage degradation. Int J Rheum Dis. 2011; 14:191–198.2. Urquhart DM, Soufan C, Teichtahl AJ, Wluka AE, Hanna F, Cicuttini FM. Factors that may mediate the relationship between physical activity and the risk for developing knee osteoarthritis. Arthritis Res Ther. 2008; 10:203.3. Aigner T. Apoptosis, necrosis, or whatever: how to find out what really happens? J Pathol. 2002; 198:1–4.4. Tuoheti Y, Itoi E, Pradhan RL, Wakabayashi I, Takahashi S, Minagawa H, Kobayashi M, Okada K, Shimada Y. Apoptosis in the supraspinatus tendon with stage II subacromial impingement. J Shoulder Elbow Surg. 2005; 14:535–541.5. Hashimoto S, Ochs RL, Komiya S, Lotz M. Linkage of chondrocyte apoptosis and cartilage degradation in human osteoarthritis. Arthritis Rheum. 1998; 41:1632–1638.6. Ea HK, Lioté F. Advances in understanding calcium-containing crystal disease. Curr Opin Rheumatol. 2009; 21:150–157.7. Jiang L, Li L, Geng C, Gong D, Jiang L, Ishikawa N, Kajima K, Zhong L. Monosodium iodoacetate induces apoptosis via the mitochondrial pathway involving ROS production and caspase activation in rat chondrocytes in vitro. J Orthop Res. 2013; 31:364–369.8. Felson DT, Zhang Y. An update on the epidemiology of knee and hip osteoarthritis with a view to prevention. Arthritis Rheum. 1998; 41:1343–1355.9. Lander HM. An essential role for free radicals and derived species in signal transduction. FASEB J. 1997; 11:118–124.10. Rasool M, Varalakshmi P. Immunomodulatory role of Withania somnifera root powder on experimental induced inflammation: an in vivo and in vitro study. Vascul Pharmacol. 2006; 44:406–410.11. Fulda S, Kroemer G. Mitochondria as therapeutic targets for the treatment of malignant disease. Antioxid Redox Signal. 2011; 15:2937–2949.12. Oh M, Fukuda K, Asada S, Yasuda Y, Tanaka S. Concurrent generation of nitric oxide and superoxide inhibits proteoglycan synthesis in bovine articular chondrocytes: involvement of peroxynitrite. J Rheumatol. 1998; 25:2169–2174.13. Hurst-Kennedy J, Boyan BD, Schwartz Z. Lysophosphatidic acid signaling promotes proliferation, differentiation, and cell survival in rat growth plate chondrocytes. Biochim Biophys Acta. 2009; 1793:836–846.14. Chen Q, Gao Y, Kao X, Chen J, Xue W, Xiong Y, Wang Z. SNP-induced apoptosis may be mediated with caspase inhibitor by JNK signaling pathways in rabbit articular chondrocytes. J Toxicol Sci. 2012; 37:157–167.15. Duranteau J, Chandel NS, Kulisz A, Shao Z, Schumacker PT. Intracellular signaling by reactive oxygen species during hypoxia in cardiomyocytes. J Biol Chem. 1998; 273:11619–11624.16. Lima D, Hermida J, Hashimoto S, Colwell C, Lotz M. Caspase inhibitors reduce severity of cartilage lesions in experimental osteoarthritis. Arthritis Rheum. 2006; 54:1814–1821.17. Salvemini D, Cuzzocrea S. Oxidative stress in septic shock and disseminated intravascular coagulation. Free Radic Biol Med. 2002; 33:1173–1185.18. Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002; 82:47–95.19. Lee HG, Yang JH. PCB126 induces apoptosis of chondrocytes via ROS-dependent pathways. Osteoarthritis Cartilage. 2012; 20:1179–1185.20. Henrotin YE, Bruckner P, Pujol JP. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthritis Cartilage. 2003; 11:747–755.21. Kim SJ, Ju JW, Oh CD, Yoon YM, Song WK, Kim JH, Yoo YJ, Bang OS, Kang SS, Chun JS. ERK-1/2 and p38 kinase oppositely regulate nitric oxide-induced apoptosis of chondrocytes in association with p53, caspase-3, and differentiation status. J Biol Chem. 2002; 277:1332–1339.22. Chuang SM, Wang IC, Yang JL. Roles of JNK, p38 and ERK mitogen-activated protein kinases in the growth inhibition and apoptosis induced by cadmium. Carcinogenesis. 2000; 21:1423–1432.23. Xu Y, Ouyang J, Zhang QG, Zhou M, Li J, Chen MM, Xu YY. Gambogic acid induces apoptosis of Jurkat cell through the MAPK signal pathway. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2012; 20:587–591.24. Hill CS, Treisman R. Transcriptional regulation by extracellular signals: mechanisms and specificity. Cell. 1995; 80:199–211.25. Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K, Gotoh Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997; 275:90–94.26. Behrens A, Sibilia M, Wagner EF. Amino-terminal phosphorylation of c-Jun regulates stress-induced apoptosis and cellular proliferation. Nat Genet. 1999; 21:326–329.27. Dai DL, Martinka M, Li G. Prognostic significance of activated Akt expression in melanoma: a clinicopathologic study of 292 cases. J Clin Oncol. 2005; 23:1473–1482.28. Kim H, Lee JU, Moon SH, Kim HC, Kwon UH, Seol NH, Kim HJ, Park JO, Chun HJ, Kwon IK, et al. Zonal responsiveness of the human intervertebral disc to bone morphogenetic protein-2. Spine (Phila Pa 1976). 2009; 34:1834–1838.29. Zhou H, Li XM, Meinkoth J, Pittman RN. Akt regulates cell survival and apoptosis at a postmitochondrial level. J Cell Biol. 2000; 151:483–494.30. Tang D, Okada H, Ruland J, Liu L, Stambolic V, Mak TW, Ingram AJ. Akt is activated in response to an apoptotic signal. J Biol Chem. 2001; 276:30461–30466.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Myricetin Inhibits Angiogenesis by Inducing Apoptosis and Suppressing PI3K/Akt/mTOR Signaling in Endothelial Cells
- Induction of Hepatocellular Carcinoma Cell Cycle Arrest and Apoptosis by Dendropanax morbifera Leveille Leaf Extract via the PI3K/AKT/mTOR Pathway
- Arctigenin Increases Hemeoxygenase-1 Gene Expression by Modulating PI3K/AKT Signaling Pathway in Rat Primary Astrocytes
- Role of Reactive Oxygen Species on Sodium Butyrate Induced Human Hepatocyte Differentiation
- Dihydroaustrasulfone alcohol induces apoptosis in nasopharyngeal cancer cells by inducing reactive oxygen speciesdependent inactivation of the PI3K/AKT pathway