J Adv Prosthodont.
2015 Apr;7(2):151-159. 10.4047/jap.2015.7.2.151.
Microscopical and chemical surface characterization of CAD/CAM zircona abutments after different cleaning procedures. A qualitative analysis
- Affiliations
-
- 1Private Practice, Ludwigshafen, Germany. dr-gehrke@prof-dhom.de
- 2Private Practice, Offenburg, Germany.
- 3Dental Laboratory, Frankfurt, Germany.
- KMID: 2118246
- DOI: http://doi.org/10.4047/jap.2015.7.2.151
Abstract
- PURPOSE
To describe and characterize the surface topography and cleanliness of CAD/CAM manufactured zirconia abutments after steaming and ultrasonic cleaning.
MATERIALS AND METHODS
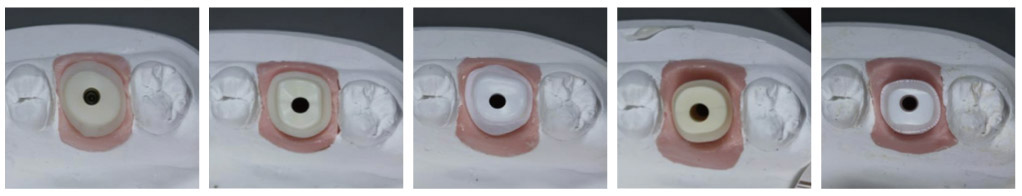
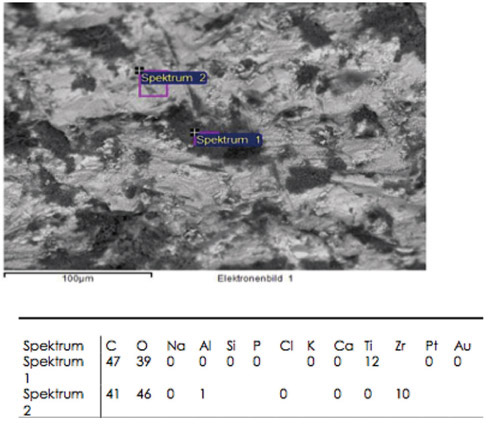
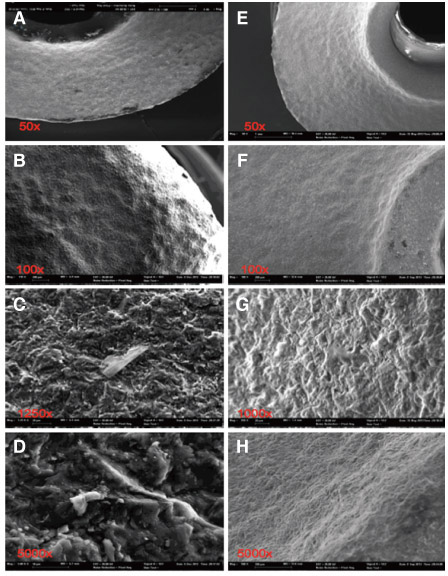
A total of 12 ceramic CAD/CAM implant abutments of various manufacturers were produced and randomly divided into two groups of six samples each (control and test group). Four two-piece hybrid abutments and two one-piece abutments made of zirconium-dioxide were assessed per each group. In the control group, cleaning by steam was performed. The test group underwent an ultrasonic cleaning procedure with acetone, ethyl alcohol and antibacterial solution. Groups were subjected to scanning electron microscope (SEM) analysis and Energy-dispersive X-ray spectroscopy (EDX) to verify and characterize contaminant chemical characterization non-quantitatively.
RESULTS
All zirconia CAD/CAM abutments in the present study displayed production-induced wear particles, debris as well as organic and inorganic contaminants. The abutments of the test group showed reduction of surface contamination after undergoing an ultrasonic cleaning procedure. However, an absolute removal of pollutants could not be achieved.
CONCLUSION
The presence of debris on the transmucosal surface of CAD/CAM zirconia abutments of various manufacturers was confirmed. Within the limits of the study design, the results suggest that a defined ultrasonic cleaning process can be advantageously employed to reduce such debris, thus, supposedly enhancing soft tissue healing. Although the adverse long-term influence of abutment contamination on the biological stability of peri-implant tissues has been evidenced, a standardized and validated polishing and cleaning protocol still has to be implemented.
Keyword
Figure
Cited by 2 articles
-
Effects of different surface treatments on the shear bond strength of veneering ceramic materials to zirconia
Adil Othman Abdullah, Yu Hui, Xudong Sun, Sarah Pollington, Fenik Kaml Muhammed, Yi Liu
J Adv Prosthodont. 2019;11(1):65-74. doi: 10.4047/jap.2019.11.1.65.Influence of scaling procedures on the integrity of titanium nitride coated CAD/CAM abutments
Peter Gehrke, Emmanouil Spanos, Carsten Fischer, Helmut Storck, Florian Tebbel, Dirk Duddeck
J Adv Prosthodont. 2018;10(3):197-204. doi: 10.4047/jap.2018.10.3.197.
Reference
-
1. Abrahamsson I, Berglundh T, Lindhe J. The mucosal barrier following abutment dis/reconnection. An experimental study in dogs. J Clin Periodontol. 1997; 24:568–572.2. Lindhe J, Berglundh T. The interface between the mucosa and the implant. Periodontol 2000. 1998; 17:47–54.3. Welander M, Abrahamsson I, Berglundh T. The mucosal barrier at implant abutments of different materials. Clin Oral Implants Res. 2008; 19:635–641.4. Buser D, Weber HP, Donath K, Fiorellini JP, Paquette DW, Williams RC. Soft tissue reactions to non-submerged unloaded titanium implants in beagle dogs. J Periodontol. 1992; 63:225–235.5. Berglundh T, Lindhe J, Jonsson K, Ericsson I. The topography of the vascular systems in the periodontal and peri-implant tissues in the dog. J Clin Periodontol. 1994; 21:189–193.6. Barboza EP, Caúla AL, Carvalho WR. Crestal bone loss around submerged and exposed unloaded dental implants: a radiographic and microbiological descriptive study. Implant Dent. 2002; 11:162–169.7. Misch CE, Dietsh-Misch F, Hoar J, Beck G, Hazen R, Misch CM. A bone quality-based implant system: first year of prosthetic loading. J Oral Implantol. 1999; 25:185–197.8. Canullo L, Micarelli C, Lembo-Fazio L, Iannello G, Clementini M. Microscopical and microbiologic characterization of customized titanium abutments after different cleaning procedures. Clin Oral Implants Res. 2014; 25:328–336.9. Canullo L, Micarelli C, Iannello G. Microscopical and chemical surface characterization of the gingival portion and connection of an internal hexagon abutment before and after different technical stages of preparation. Clin Oral Implants Res. 2013; 24:606–611.10. Meyle J, Gültig K, Nisch W. Variation in contact guidance by human cells on a microstructured surface. J Biomed Mater Res. 1995; 29:81–88.11. Grössner-Schreiber B, Herzog M, Hedderich J, Dück A, Hannig M, Griepentrog M. Focal adhesion contact formation by fibroblasts cultured on surface-modified dental implants: an in vitro study. Clin Oral Implants Res. 2006; 17:736–745.12. Teughels W, Van Assche N, Sliepen I, Quirynen M. Effect of material characteristics and/or surface topography on biofilm development. Clin Oral Implants Res. 2006; 17:68–81.13. van Winkelhoff AJ, Goené RJ, Benschop C, Folmer T. Early colonization of dental implants by putative periodontal pathogens in partially edentulous patients. Clin Oral Implants Res. 2000; 11:511–520.14. Quirynen M, Bollen CM. The influence of surface roughness and surface-free energy on supra- and subgingival plaque formation in man. A review of the literature. J Clin Periodontol. 1995; 22:1–14.15. Quirynen M, Bollen CM, Papaioannou W, Van Eldere J, van Steenberghe D. The influence of titanium abutment surface roughness on plaque accumulation and gingivitis: short-term observations. Int J Oral Maxillofac Implants. 1996; 11:169–178.16. Fürst MM, Salvi GE, Lang NP, Persson GR. Bacterial colonization immediately after installation on oral titanium implants. Clin Oral Implants Res. 2007; 18:501–508.17. Salvi GE, Fürst MM, Lang NP, Persson GR. One-year bacterial colonization patterns of Staphylococcus aureus and other bacteria at implants and adjacent teeth. Clin Oral Implants Res. 2008; 19:242–248.18. Mishra PK, Wu W, Rozo C, Hallab NJ, Benevenia J, Gause WC. Micrometer-sized titanium particles can induce potent Th2-type responses through TLR4-independent pathways. J Immunol. 2011; 187:6491–6498.19. Piattelli A, Pontes AE, Degidi M, Iezzi G. Histologic studies on osseointegration: soft tissues response to implant surfaces and components. A review. Dent Mater. 2011; 27:53–60.20. Bentley EM. The value of ultrasonic cleaners in dental practice. Br Dent J. 1994; 177:53–56.21. Jatzwauk L, Schöne H, Pietsch H. How to improve instrument disinfection by ultrasound. J Hosp Infect. 2001; 48:S80–S83.22. Sawase T, Wennerberg A, Hallgren C, Albrektsson T, Baba K. Chemical and topographical surface analysis of five different implant abutments. Clin Oral Implants Res. 2000; 11:44–50.23. Canullo L, Penarrocha D, Micarelli C, Massidda O, Bazzoli M. Hard tissue response to argon plasma cleaning/sterilisation of customised titanium abutments versus 5-second steam cleaning: results of a 2-year post-loading follow-up from an explanatory randomised controlled trial in periodontally healthy patients. Eur J Oral Implantol. 2013; 6:251–260.24. Canullo L, Peñarrocha D, Clementini M, Iannello G, Micarelli C. Impact of plasma of argon cleaning treatment on implant abutments in patients with a history of periodontal disease and thin biotype: radiographic results at 24-month follow-up of a RCT. Clin Oral Implants Res. 2015; 26:8–14.25. Linkevicius T, Apse P. Influence of abutment material on stability of peri-implant tissues: a systematic review. Int J Oral Maxillofac Implants. 2008; 23:449–456.26. Nakamura K, Kanno T, Milleding P, Ortengren U. Zirconia as a dental implant abutment material: a systematic review. Int J Prosthodont. 2010; 23:299–309.27. Zembic A, Sailer I, Jung RE, Hämmerle CH. Randomizedcontrolled clinical trial of customized zirconia and titanium implant abutments for single-tooth implants in canine and posterior regions: 3-year results. Clin Oral Implants Res. 2009; 20:802–808.28. Sailer I, Philipp A, Zembic A, Pjetursson BE, Hämmerle CH, Zwahlen M. A systematic review of the performance of ceramic and metal implant abutments supporting fixed implant reconstructions. Clin Oral Implants Res. 2009; 20:4–31.29. Garbelotto LG, Maziero Volpato CA, Rocha Md, Maranghello CA, Calasans A, Ozcan M. Laboratory and clinical considerations on prosthetic zirconia infrastructures for implants. Implant Dent. 2013; 22:578–583.30. Gehrke P, Alius J, Fischer C, Erdelt KJ, Beuer F. Retentive strength of two-piece CAD/CAM zirconia implant abutments. Clin Implant Dent Relat Res. 2014; 16:920–925.31. Sailer I, Sailer T, Stawarczyk B, Jung RE, Hämmerle CH. In vitro study of the influence of the type of connection on the fracture load of zirconia abutments with internal and external implant-abutment connections. Int J Oral Maxillofac Implants. 2009; 24:850–858.32. Stimmelmayr M, Edelhoff D, Güth JF, Erdelt K, Happe A, Beuer F. Wear at the titanium-titanium and the titanium-zirconia implant-abutment interface: a comparative in vitro study. Dent Mater. 2012; 28:1215–1220.33. Truninger TC, Stawarczyk B, Leutert CR, Sailer TR, Hämmerle CH, Sailer I. Bending moments of zirconia and titanium abutments with internal and external implant-abutment connections after aging and chewing simulation. Clin Oral Implants Res. 2012; 23:12–18.34. Bollen CM, Papaioanno W, Van Eldere J, Schepers E, Quirynen M, van Steenberghe D. The influence of abutment surface roughness on plaque accumulation and peri-implant mucositis. Clin Oral Implants Res. 1996; 7:201–211.35. Butz F, Heydecke G, Okutan M, Strub JR. Survival rate, fracture strength and failure mode of ceramic implant abutments after chewing simulation. J Oral Rehabil. 2005; 32:838–843.36. Degidi M, Artese L, Scarano A, Perrotti V, Gehrke P, Piattelli A. Inflammatory infiltrate, microvessel density, nitric oxide synthase expression, vascular endothelial growth factor expression, and proliferative activity in peri-implant soft tissues around titanium and zirconium oxide healing caps. J Periodontol. 2006; 77:73–80.37. Glauser R, Sailer I, Wohlwend A, Studer S, Schibli M, Schärer P. Experimental zirconia abutments for implant-supported single-tooth restorations in esthetically demanding regions: 4-year results of a prospective clinical study. Int J Prosthodont. 2004; 17:285–290.38. Canullo L, Micarelli C, Lembo-Fazio L, Iannello G, Clementini M. Microscopical and microbiologic characterization of customized titanium abutments after different cleaning procedures. Clin Oral Implants Res. 2014; 25:328–336.39. Micarelli C, Canullo L, Baldissara P, Clementini M. Implant abutment screw reverse torque values before and after plasma cleaning. Int J Prosthodont. 2013; 26:331–333.40. Albrektsson T, Wennerberg A. Oral implant surfaces: Part 1--review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int J Prosthodont. 2004; 17:536–543.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Considerations for Fabrication of CAD-CAM Abutments: Part I. Selection of Titanium Block and Fabrication Process
- Microbiological cleaning and disinfection efficacy of a three-stage ultrasonic processing protocol for CAD-CAM implant abutments
- Chairside computer-aided design/computer-aided manufacturing (CAD/ CAM)-based restoration of anterior teeth with customized shade and surface characterization: a report of 2 cases
- Comparison of CAD/CAM abutment and prefabricated abutment in Morse taper internal type implant after cyclic loading: Axial displacement, removal torque, and tensile removal force
- Influence of scaling procedures on the integrity of titanium nitride coated CAD/CAM abutments