J Adv Prosthodont.
2018 Jun;10(3):197-204. 10.4047/jap.2018.10.3.197.
Influence of scaling procedures on the integrity of titanium nitride coated CAD/CAM abutments
- Affiliations
-
- 1Private Practice, Ludwigshafen, Germany. dr-gehrke@prof-dhom.de
- 2Private Practice, Sulzheim, Germany.
- 3Dental Laboratory, Sirius Ceramics, Frankfurt am Main, Germany.
- 4Dental Laboratory, LUSANUM, Ludwigshafen, Germany.
- 5Mechanical Engineering, Mannheim, Germany.
- 6Medical Materials Research Institute, Berlin, Germany.
- KMID: 2413488
- DOI: http://doi.org/10.4047/jap.2018.10.3.197
Abstract
- PURPOSE
To determine the extent of treatment traces, the roughness depth, and the quantity of titanium nitride (TiN) removed from the surface of CAD/CAM abutments after treatment with various instruments.
MATERIALS AND METHODS
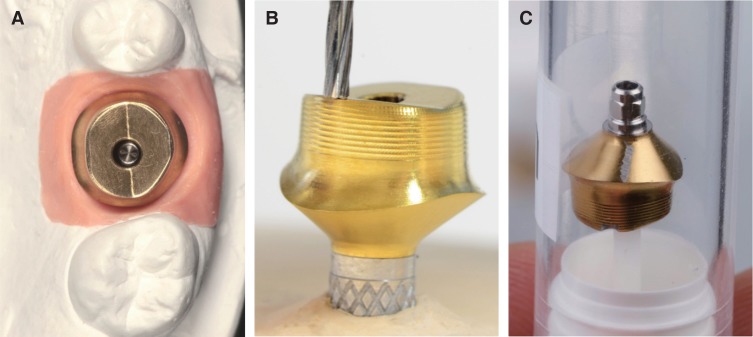
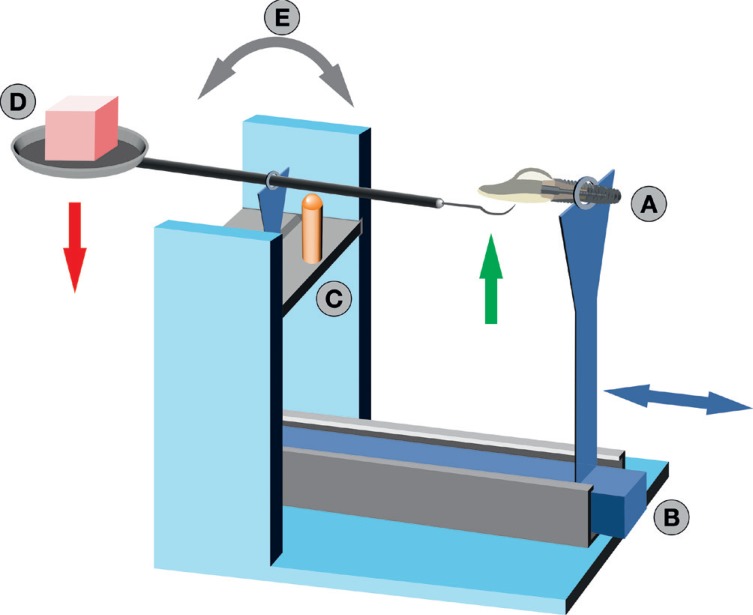
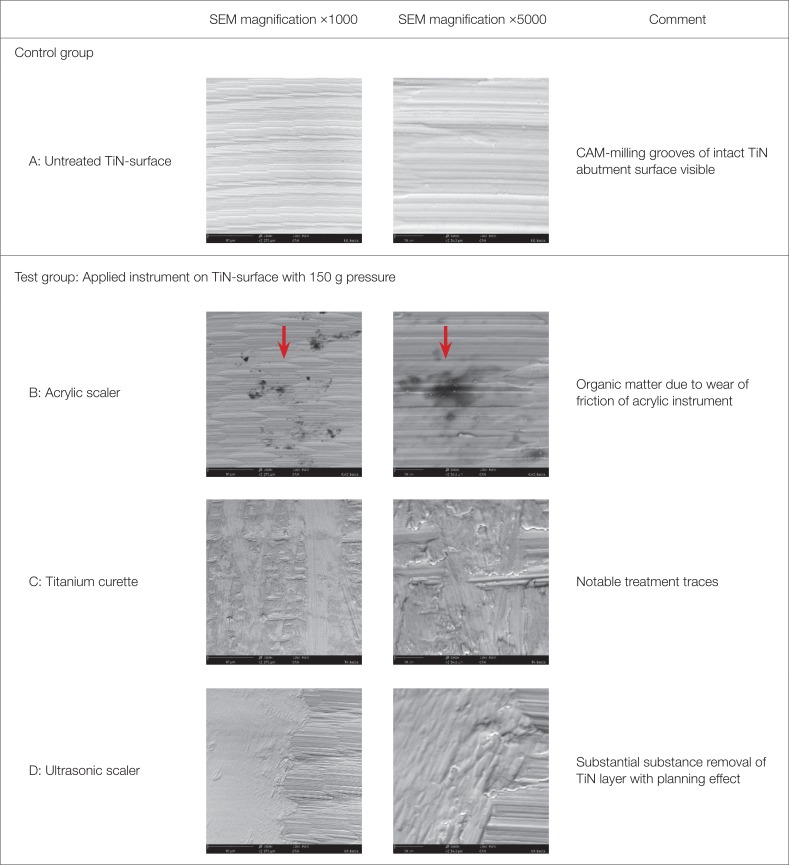
Twelve TiN coated CAD/CAM abutments were investigated for an in vitro study. In the test group (9), each abutment surface was subjected twice (150 g vs. 200 g pressure) to standardized treatment in a simulated prophylaxis measure with the following instruments: acrylic scaler, titanium curette, and ultrasonic scaler with steel tip. Three abutments were used as control group. Average surface roughness (Sa) and developed interfacial area ratio (Sdr) of treated and untreated surfaces were measured with a profilometer. The extent of treatment traces were analyzed by scanning electron microscopy.
RESULTS
Manipulation with ultrasonic scalers resulted in a significant increase of average surface roughness (Sa, P < .05) and developed interfacial area ratio (Sdr, P < .018). Variable contact pressure did not yield any statistically significant difference on Sa-values for all instruments (P=.8). Ultrasonic treatment resulted in pronounced surface traces and partially detachment of the TiN coating. While titanium curettes caused predominantly moderate treatment traces, no traces or detectable substance removal has been determined after manipulation with acrylic curettes.
CONCLUSION
Inappropriate instruments during regular plaque control may have an adverse effect on the integrity of the TiN coating of CAD/CAM abutments. To prevent defects and an increased surface roughness at the transmucosal zone of TiN abutments, only acrylic scaling instruments can be recommended for regular maintenance care.
MeSH Terms
Figure
Reference
-
1. Sala L, Bascones-Martínez A, Carrillo-de-Albornoz A. Impact of abutment material on peri-implant soft tissue color. An in vitro study. Clin Oral Investig. 2017; 21:2221–2233.
Article2. Sicilia A, Quirynen M, Fontolliet A, Francisco H, Friedman A, Linkevicius T, Lutz R, Meijer HJ, Rompen E, Rotundo R, Schwarz F, Simion M, Teughels W, Wennerberg A, Zuhr O. Long-term stability of peri-implant tissues after bone or soft tissue augmentation. Effect of zirconia or titanium abutments on peri-implant soft tissues. Summary and consensus statements. The 4th EAO Consensus Conference 2015. Clin Oral Implants Res. 2015; 26:148–152. PMID: 26385628.
Article3. Sailer I, Zembic A, Jung RE, Hämmerle CH, Mattiola A. Single-tooth implant reconstructions: esthetic factors influencing the decision between titanium and zirconia abutments in anterior regions. Eur J Esthet Dent. 2007; 2:296–310. PMID: 19655552.4. Lops D, Bressan E, Parpaiola A, Sbricoli L, Cecchinato D, Romeo E. Soft tissues stability of cad-cam and stock abutments in anterior regions: 2-year prospective multicentric cohort study. Clin Oral Implants Res. 2015; 26:1436–1442. PMID: 25196805.
Article5. Park SE, Da Silva JD, Weber HP, Ishikawa-Nagai S. Optical phenomenon of peri-implant soft tissue. Part I. Spectrophotometric assessment of natural tooth gingiva and peri-implant mucosa. Clin Oral Implants Res. 2007; 18:569–574. PMID: 17655713.
Article6. Ioannidis A, Cathomen E, Jung RE, Fehmer V, Hüsler J, Thoma DS. Discoloration of the mucosa caused by different restorative materials - a spectrophotometric in vitro study. Clin Oral Implants Res. 2017; 28:1133–1138. PMID: 27452796.7. Bittencourt TC, Ribeiro CG, Devito KL, Ferreira CF, Cagna DR, Picorelli NM. Zirconia abutment supporting all ceramic crowns in the esthetic zone: Interim results of a prospective study. Eur J Prosthodont Restor Dent. 2016; 24:23–30. PMID: 27039475.8. Zembic A, Sailer I, Jung RE, Hämmerle CH. Randomized-controlled clinical trial of customized zirconia and titanium implant abutments for single-tooth implants in canine and posterior regions: 3-year results. Clin Oral Implants Res. 2009; 20:802–808. PMID: 19486077.
Article9. Degidi M, Artese L, Scarano A, Perrotti V, Gehrke P, Piattelli A. Inflammatory infiltrate, microvessel density, nitric oxide synthase expression, vascular endothelial growth factor expression, and proliferative activity in peri-implant soft tissues around titanium and zirconium oxide healing caps. J Periodontol. 2006; 77:73–80. PMID: 16579706.
Article10. Nakamura K, Kanno T, Milleding P, Ortengren U. Zirconia as a dental implant abutment material: a systematic review. Int J Prosthodont. 2010; 23:299–309. PMID: 20617217.11. Brodbeck U. The ZiReal Post: A new ceramic implant abutment. J Esthet Restor Dent. 2003; 15:10–23. PMID: 12638769.
Article12. Coray R, Zeltner M, Özcan M. Fracture strength of implant abutments after fatigue testing: A systematic review and a meta-analysis. J Mech Behav Biomed Mater. 2016; 62:333–346. PMID: 27239815.
Article13. Gehrke P, Johannson D, Fischer C, Stawarczyk B, Beuer F. In vitro fatigue and fracture resistance of one- and two-piece CAD/CAM zirconia implant abutments. Int J Oral Maxillofac Implants. 2015; 30:546–554. PMID: 26009905.
Article14. Garine WN, Funkenbusch PD, Ercoli C, Wodenscheck J, Murphy WC. Measurement of the rotational misfit and implant-abutment gap of all-ceramic abutments. Int J Oral Maxillofac Implants. 2007; 22:928–938. PMID: 18271374.15. Ferrari M, Carrabba M, Vichi A, Goracci C, Cagidiaco MC. Influence of abutment color and mucosal thickness on soft tissue color. Int J Oral Maxillofac Implants. 2017; 32:393–399. PMID: 27525517.
Article16. Ferrari M, Tricarico MG, Cagidiaco MC, Vichi A, Gherlone EF, Zarone F, Sorrentino R. 3-year randomized controlled prospective clinical trial on different CAD-CAM implant abutments. Clin Implant Dent Relat Res. 2016; 18:1134–1141. PMID: 26988025.
Article17. Scarano A, Piattelli M, Vrespa G, Caputi S, Piattelli A. Bacterial adhesion on titanium nitride-coated and uncoated implants: an in vivo human study. J Oral Implantol. 2003; 29:80–85. PMID: 12760451.
Article18. Annunziata M, Oliva A, Basile MA, Giordano M, Mazzola N, Rizzo A, Lanza A, Guida L. The effects of titanium nitride-coating on the topographic and biological features of TPS implant surfaces. J Dent. 2011; 39:720–728. PMID: 21856369.
Article19. Vaz F, Cerqueira P, Rebouta L, Nascimento SMC, Alves E, Goudeau P, Rivière JP, Pischow K, de Rijk J. Structural, optical and mechanical properties of coloured TiNxOy thin films. Thin Solid Films [Internet]. Elsevier BV;2004. 447-8:p. 449–454. Available from: http://dx.doi.org/10.1016/s0040-6090(03)01123-4.20. Lops D, Stellini E, Sbricoli L, Cea N, Romeo E, Bressan E. Influence of abutment material on peri-implant soft tissues in anterior areas with thin gingival biotype: a multicentric prospective study. Clin Oral Implants Res. 2017; 28:1263–1268. PMID: 27699895.
Article21. Ferrari M, Cagidiaco MC, Garcia-Godoy F, Goracci C, Cairo F. Effect of different prosthetic abutments on peri-implant soft tissue. A randomized controlled clinical trial. Am J Dent. 2015; 28:85–89. PMID: 26087573.22. Groessner-Schreiber B, Hannig M, Dück A, Griepentrog M, Wenderoth DF. Do different implant surfaces exposed in the oral cavity of humans show different biofilm compositions and activities? Eur J Oral Sci. 2004; 112:516–522. PMID: 15560835.
Article23. Kim YS, Shin SY, Moon SK, Yang SM. Surface properties correlated with the human gingival fibroblasts attachment on various materials for implant abutments: a multiple regression analysis. Acta Odontol Scand. 2015; 73:38–47. PMID: 25183254.
Article24. Chien CC, Liu KT, Duh JG, Chang KW, Chung KH. Effect of nitride film coatings on cell compatibility. Dent Mater. 2008; 24:986–993. PMID: 18177932.
Article25. Bollen CM, Papaioanno W, Van Eldere J, Schepers E, Quirynen M, van Steenberghe D. The influence of abutment surface roughness on plaque accumulation and peri-implant mucositis. Clin Oral Implants Res. 1996; 7:201–211. PMID: 9151584.
Article26. Mehl C, Kern M, Schütte AM, Kadem LF, Selhuber-Unkel C5. Adhesion of living cells to abutment materials, dentin, and adhesive luting cement with different surface qualities. Dent Mater. 2016; 32:1524–1535. PMID: 27717514.
Article27. Grössner-Schreiber B, Griepentrog M, Haustein I, Müller WD, Lange KP, Briedigkeit H, Göbel UB. Plaque formation on surface modified dental implants. An in vitro study. Clin Oral Implants Res. 2001; 12:543–551. PMID: 11737097.28. Mengel R, Meer C, Flores-de-Jacoby L. The treatment of uncoated and titanium nitride-coated abutments with different instruments. Int J Oral Maxillofac Implants. 2004; 19:232–238. PMID: 15101595.29. Gehrke P, Tabellion A, Fischer C. Microscopical and chemical surface characterization of CAD/CAM zircona abutments after different cleaning procedures. A qualitative analysis. J Adv Prosthodont. 2015; 7:151–159. PMID: 25932314.
Article30. Rompen E, Domken O, Degidi M, Pontes AE, Piattelli A. The effect of material characteristics, of surface topography and of implant components and connections on soft tissue integration: a literature review. Clin Oral Implants Res. 2006; 17:55–67. PMID: 16968382.
Article31. Louropoulou A, Slot DE, Van der Weijden F. The effects of mechanical instruments on contaminated titanium dental implant surfaces: a systematic review. Clin Oral Implants Res. 2014; 25:1149–1160. PMID: 23834327.
Article32. Schwarz F, Papanicolau P, Rothamel D, Beck B, Herten M, Becker J. Influence of plaque biofilm removal on reestablishment of the biocompatibility of contaminated titanium surfaces. J Biomed Mater Res A. 2006; 77:437–444. PMID: 16444683.
Article33. Yang SM, Park JB, Ko Y. Use of confocal microscopy for quantification of plastic remnants on rough titanium after instrumentation and evaluation of efficacy of removal. Int J Oral Maxillofac Implants. 2015; 30:519–525. PMID: 26009902.
Article34. Schmage P, Kahili F, Nergiz I, Scorziello TM, Platzer U, Pfeiffer P. Cleaning effectiveness of implant prophylaxis instruments. Int J Oral Maxillofac Implants. 2014; 29:331–337. PMID: 24683558.
Article35. Schmidt KE, Auschill TM, Heumann C, Frankenberger R, Eick S, Sculean A, Arweiler NB. Influence of different instrumentation modalities on the surface characteristics and biofilm formation on dental implant neck, in vitro. Clin Oral Implants Res. 2017; 28:483–490. PMID: 27000771.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Considerations for Fabrication of CAD-CAM Abutments: Part I. Selection of Titanium Block and Fabrication Process
- Mechanical Complications of Posterior Implant Restorations using CAD/CAM Titanium Customized Abutments: A Retrospective Study
- A comparative study of gold UCLA-type and CAD/CAM titanium implant abutments
- Zirconia ceramic fixed dental prosthesis with all-on-4 concept implants for irradiated maxilla: A case report
- The use of computer-aided design and computer-aided manufacturing fabricated titanium prosthesis to restore maxillofacial defects: 4 cases report