J Korean Soc Magn Reson Med.
2014 Jun;18(2):120-132. 10.13104/jksmrm.2014.18.2.120.
Differentiation of True Recurrence from Delayed Radiation Therapy-related Changes in Primary Brain Tumors Using Diffusion-weighted Imaging, Dynamic Susceptibility Contrast Perfusion Imaging, and Susceptibility-weighted Imaging
- Affiliations
-
- 1Department of Radiology, Seoul National University College of Medicine, Seoul, Korea. verocay@snuh.org
- 2Center for Nanoparticle Research, Institute for Basic Science, and School of Chemical and Biological Engineering, Seoul National University, Seoul, Korea.
- 3Department of Internal Medicine, Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- 4Department of Neurosurgery, Seoul National University College of Medicine, Seoul, Korea.
- 5Department of Pathology, Seoul National University College of Medicine, Seoul, Korea.
- 6Department of Radiation Oncology, Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2099888
- DOI: http://doi.org/10.13104/jksmrm.2014.18.2.120
Abstract
- PURPOSE
To compare dynamic susceptibility contrast imaging, diffusion-weighted imaging, and susceptibility-weighted imaging (SWI) for the differentiation of tumor recurrence and delayed radiation therapy (RT)-related changes in patients treated with RT for primary brain tumors.
MATERIALS AND METHODS
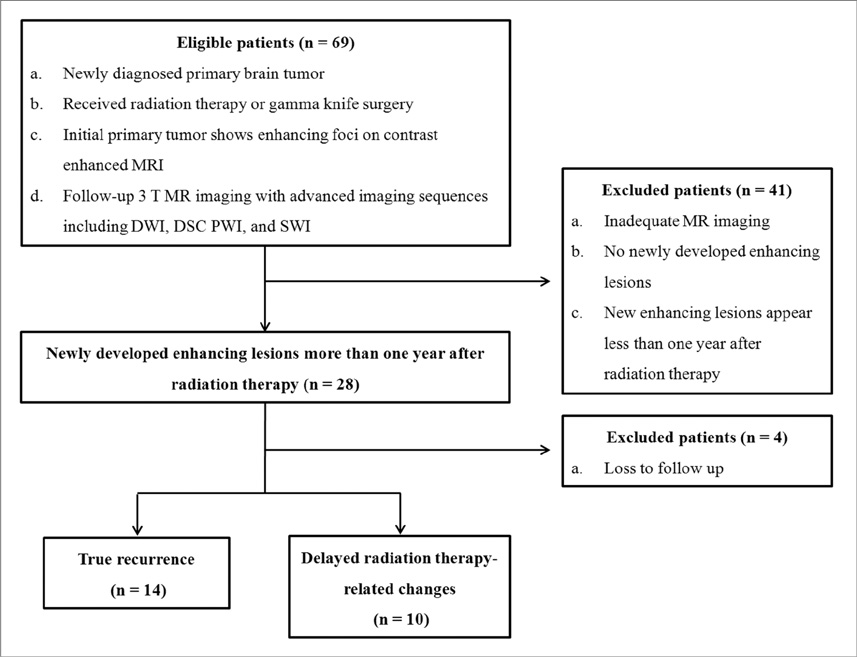
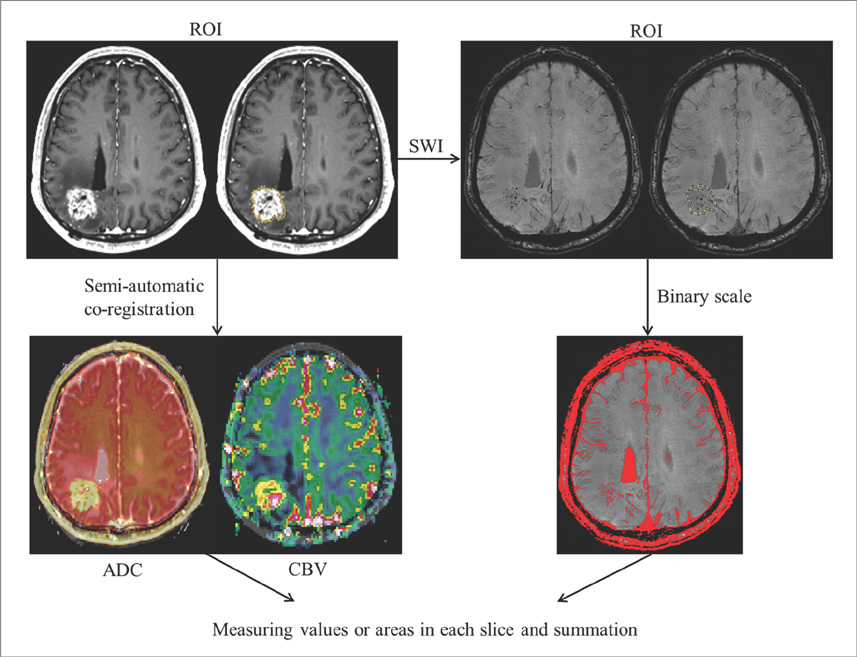
We enrolled 24 patients treated with RT for various primary brain tumors, who showed newly appearing enhancing lesions more than one year after completion of RT on follow-up MRI. The enhancing-lesions were confirmed as recurrences (n=14) or RT-changes (n=10). We calculated the mean values of normalized cerebral blood volume (nCBV), apparent diffusion coefficient (ADC), and proportion of dark signal intensity on SWI (proSWI) for the enhancing-lesions. All the values between the two groups were compared using t-test. A multivariable logistic regression model was used to determine the best predictor of differential diagnosis. The cutoff value of the best predictor obtained from receiver-operating characteristic curve analysis was applied to calculate the sensitivity, specificity, and accuracy for the diagnosis.
RESULTS
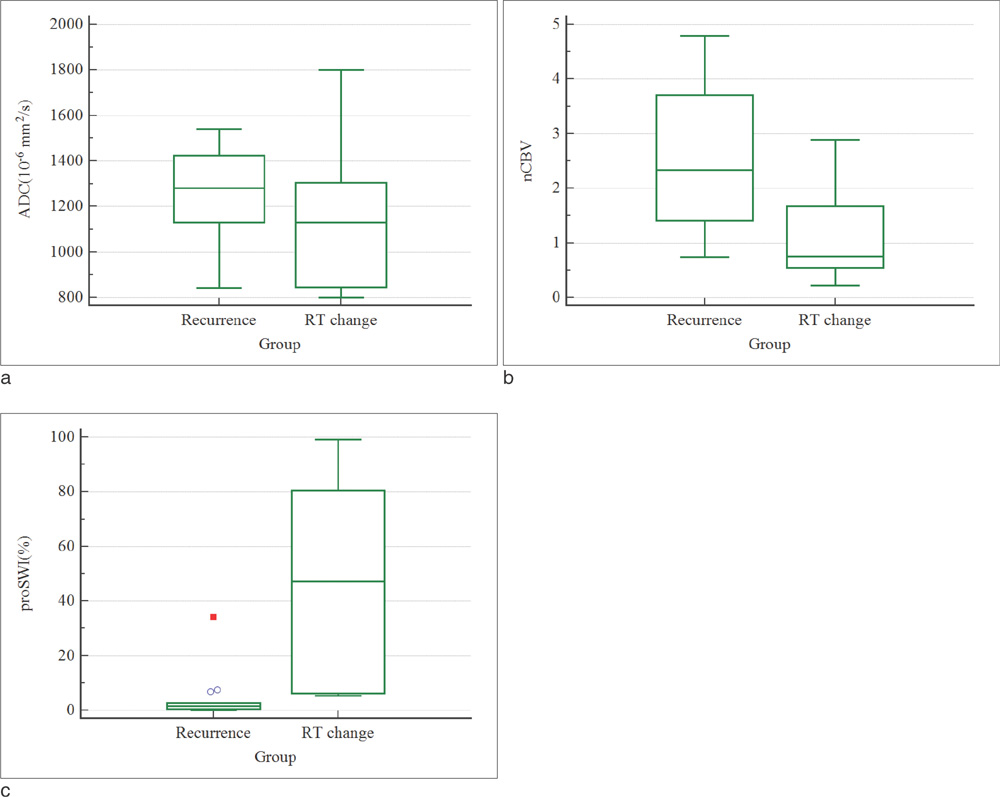
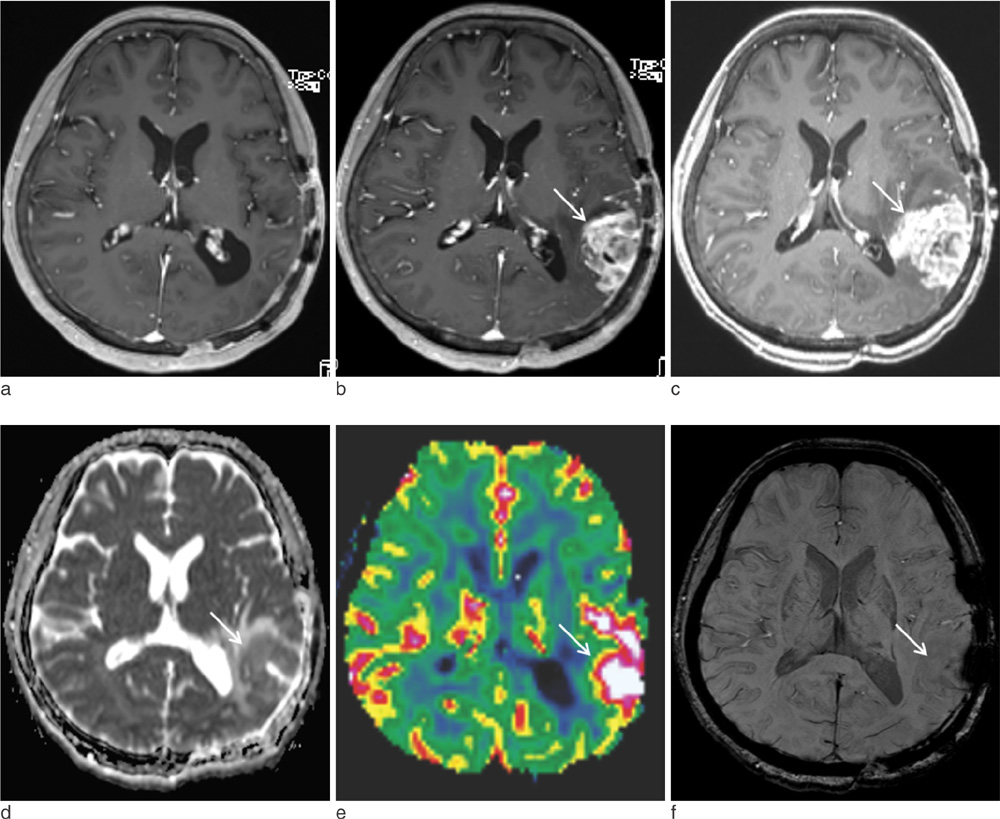
The mean nCBV value was significantly higher in the recurrence group than in the RT-change group (P=.004), and the mean proSWI was significantly lower in the recurrence group (P<.001). However, no significant difference was observed in the mean ADC values between the two groups. A multivariable logistic regression analysis showed that proSWI was the only independent variable for the differentiation; the sensitivity, specificity, and accuracy were 78.6% (11 of 14), 100% (10 of 10), and 87.5% (21 of 24), respectively.
CONCLUSION
The proSWI was the most promising parameter for the differentiation of newly developed enhancing-lesions more than one year after RT completion in brain tumor patients.
Keyword
MeSH Terms
Figure
Reference
-
1. Wang YX, King AD, Zhou H, et al. Evolution of radiation-induced brain injury: MR imaging-based study. Radiology. 2010; 254:210–218.2. Mullins ME, Barest GD, Schaefer PW, Hochberg FH, Gonzalez RG, Lev MH. Radiation necrosis versus glioma recurrence: conventional MR imaging clues to diagnosis. AJNR Am J Neuroradiol. 2005; 26:1967–1972.3. Kim HS, Kim JH, Kim SH, Cho KG, Kim SY. Posttreatment high-grade glioma: usefulness of peak height position with semiquantitative MR perfusion histogram analysis in an entire contrast-enhanced lesion for predicting volume fraction of recurrence. Radiology. 2010; 256:906–915.4. Chan YL, Leung SF, King AD, Choi PH, Metreweli C. Late radiation injury to the temporal lobes: morphologic evaluation at MR imaging. Radiology. 1999; 213:800–807.5. Valk PE, Dillon WP. Radiation injury of the brain. AJNR Am J Neuroradiol. 1991; 12:45–62.6. Dooms GC, Hecht S, Brant-Zawadzki M, Berthiaume Y, Norman D, Newton TH. Brain radiation lesions: MR imaging. Radiology. 1986; 158:149–155.7. Curran WJ, Hecht-Leavitt C, Schut L, Zimmerman RA, Nelson DF. Magnetic resonance imaging of cranial radiation lesions. Int J Radiat Oncol Biol Phys. 1987; 13:1093–1098.8. Kim YH, Oh SW, Lim YJ, et al. Differentiating radiation necrosis from tumor recurrence in highgrade gliomas: assessing the efficacy of 18F-FDG PET, 11C-methionine PET and perfusion MRI. Clin Neurol Neurosurg. 2010; 112:758–765.9. Castillo M, Smith JK, Kwock L, Wilber K. Apparent diffusion coefficients in the evaluation of high-grade gliomas. AJNR Am J Neuroradiol. 2001; 22:60–64.10. Sugahara T, Korogi Y, Kochi M, et al. Usefulness of diffusion weighted MRI with echo-planar technique in the evaluation of cellularity in gliomas. J Magn Reson Imaging. 1999; 9:53–60.11. Hein PA, Eskey CJ, Dunn JF, Hug EB. Diffusion-weighted imaging in the follow-up of treated high-grade gliomas: tumor recurrence versus radiation injury. AJNR Am J Neuroradiol. 2004; 25:201–209.12. Asao C, Korogi Y, Kitajima M, et al. Diffusion-weighted imaging of radiation-induced brain injury for differentiation from tumor recurrence. AJNR Am J Neuroradiol. 2005; 26:1455–1460.13. Larsen VA, Simonsen HJ, Law I, Larsson HB, Hansen AE. Evaluation of dynamic contrast-enhanced T1-weighted perfusion MRI in the differentiation of tumor recurrence from radiation necrosis. Neuroradiology. 2013; 55:361–369.14. Sugahara T, Korogi Y, Tomiguchi S, et al. Posttherapeutic intraaxial brain tumor: the value of perfusion-sensitive contrast-enhanced MR imaging for differentiating tumor recurrence from nonneoplastic contrast-enhancing tissue. AJNR Am J Neuroradiol. 2000; 21:901–909.15. Covarrubias DJ, Rosen BR, Lev MH. Dynamic magnetic resonance perfusion imaging of brain tumors. Oncologist. 2004; 9:528–537.16. Thomas B, Somasundaram S, Thamburaj K, et al. Clinical applications of susceptibility weighted MR imaging of the brain - a pictorial review. Neuroradiology. 2008; 50:105–116.17. Crossen JR, Garwood D, Glatstein E, Neuwelt EA. Neurobehavioral sequelae of cranial irradiation in adults: a review of radiation-induced encephalopathy. J Clin Oncol. 1994; 12:627–642.18. Giglio P, Gilbert MR. Cerebral radiation necrosis. Neurologist. 2003; 9:180–188.19. Heckl S, Aschoff A, Kunze S. Radiation-induced cavernous hemangiomas of the brain: a late effect predominantly in children. Cancer. 2002; 94:3285–3291.20. Burn S, Gunny R, Phipps K, Gaze M, Hayward R. Incidence of cavernoma development in children after radiotherapy for brain tumors. J Neurosurg. 2007; 106:379–383.21. Sheline GE. Radiation therapy of brain tumors. Cancer. 1977; 39:873–881.22. Rosen BR, Belliveau JW, Vevea JM, Brady TJ. Perfusion imaging with NMR contrast agents. Magn Reson Med. 1990; 14:249–265.23. Ostergaard L, Weisskoff RM, Chesler DA, Gyldensted C, Rosen BR. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part I: mathematical approach and statistical analysis. Magn Reson Med. 1996; 36:715–772.24. Boxerman JL, Schmainda KM, Weisskoff RM. Relative cerebral blood volume maps corrected for contrast agent extravasation significantly correlate with glioma tumor grade, whereas uncorrected maps do not. AJNR Am J Neuroradiol. 2006; 27:859–867.25. Wetzel SG, Cha S, Johnson G, et al. Relative cerebral blood volume measurements in intracranial mass lesions: interobserver and intraobserver reproducibility study. Radiology. 2002; 224:797–803.26. Hauck WW, Miike R. A proposal for examining and reporting stepwise regressions. Stat Med. 1991; 10:711–715.27. Rosen BR, Belliveau JW, Vevea JM, Brady TJ. Perfusion imaging with NMR contrast agents. Magn Reson Med. 1990; 14:249–265.28. Hoefnagels FW, Lagerwaard FJ, Sanchez E, et al. Radiological progression of cerebral metastases after radiosurgery: assessment of perfusion MRI for differentiating between necrosis and recurrence. J Neurol. 2009; 256:878–887.29. Barajas RF, Chang JS, Sneed PK, Segal MR, McDermott MW, Cha S. Distinguishing recurrent intra-axial metastatic tumor from radiation necrosis following gamma knife radiosurgery using dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. AJNR Am J Neuroradiol. 2009; 30:367–372.30. Hu LS, Baxter LC, Smith KA, et al. Relative cerebral blood volume values to differentiate high-grade glioma recurrence from posttreatment radiation effect: direct correlation between image-guided tissue histopathology and localized dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging measurements. AJNR Am J Neuroradiol. 2009; 30:552–558.31. Gasparetto EL, Pawlak MA, Patel SH, et al. Posttreatment recurrence of malignant brain neoplasm: accuracy of relative cerebral blood volume fraction in discriminating low from high malignant histologic volume fraction. Radiology. 2009; 250:887–896.32. Stadnik TW, Chaskis C, Michotte A, et al. Diffusion-weighted MR imaging of intracerebral masses: comparison with conventional MR imaging and histologic findings. AJNR Am J Neuroradiol. 2001; 22:969–976.33. Schaefer PW, Ozsunar Y, He J, et al. Assessing tissue viability with MR diffusion and perfusion imaging. AJNR Am J Neuroradiol. 2003; 24:436–443.34. Guo AC, Cummings TJ, Dash RC, Provenzale JM. Lymphomas and high-grade astrocytomas: comparison of water diffusibility and histologic characteristics. Radiology. 2002; 224:177–183.35. Matsusue E, Fink JR, Rockhill JK, Ogawa T, Maravilla KR. Distinction between glioma progression and post-radiation change by combined physiologic MRimaging. Neuroradiology. 2010; 52:297–306.36. Tung GA, Evangelista P, Rogg JM, Duncan JA. Diffusion-weighted MR imaging of rim-enhancing brain masses: is markedly decreased water diffusion specific for brain abscess? AJR Am J Roentgenol. 2001; 177:709–712.37. Holtas S, Geijer B, Stromblad LG, Mary-Sundgren P, Burtscher IM. A ring-enhancing metastasis with central high signal on diffusion-weighted Imaging and low apparent diffusion coefficients. Neuroradiology. 2000; 42:824–827.38. Biousse V, Newman NJ, Hunter SB, Hudgins PA. Diffusion weighted imaging in radiation necrosis. J Neurol Neurosurg Psychiatry. 2003; 74:382–384.39. Burger PC, Boyko OB. The pathology of central nervous system radiation injury. In : Gutin PH, Leibel SA, Sheline GE, editors. Radiation Injury to the Central Nervous System. New York, NY: Raven;1991. p. 191–208.40. Silvera S, Oppenheim C, Touzé E, et al. Spontaneous intracerebral hematoma on diffusion-weighted images: influence of T2-shine-through and T2-blackout effects. AJNR Am J Neuroradiol. 2005; 26:236–241.41. Gaensler EH, Dillon WP, Edwards MS, Larson DA, Rosenau W, Wilson CB. Radiation-induced telangiectasia in the brain simulates cryptic vascular malformations at MR imaging. Radiology. 1994; 193:629–636.42. Zeng QS, Kang XS, Li CF, Zhou GY. Detection of hemorrhagic hypointense foci in radiation injury region using susceptibility-weighted imaging. Acta Radiol. 2011; 52:115–119.43. Poussaint TY, Siffert J, Barnes PD, et al. Hemorrhagic vasculopathy after treatment of central nervous system neoplasia in childhood: diagnosis and follow-up. AJNR Am J Neuroradiol. 1995; 16:693–699.44. Llena JF, Cespedes G, Hirano A, Zimmerman HM, Feiring EH, Fine D. Vascular alterations in delayed radiation necrosis of the brain. An electron microscopical study. Arch Pathol Lab Med. 1976; 100:531–534.45. Okeda R, Shibata T. Radiation encephalopathy: an autopsy case and some comments on the pathogenesis of delayed radionecrosis of the central nervous system. Acta Pathol Jpn. 1973; 23:867–883.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Advanced Magnetic Resonance Imaging for Pediatric Brain Tumors: Current Imaging Techniques and Interpretation Algorithms
- Advanced MRI for Pediatric Brain Tumors with Emphasis on Clinical Benefits
- Arterial Spin Labelling Perfusion, Proton MR Spectroscopy and Susceptibility-Weighted MR Findings of Acute Necrotizing Encephalopathy: a Case Report
- Current Applications and Future Perspectives of Brain Tumor Imaging
- Basics for Pediatric Brain Tumor Imaging: Techniques and Protocol Recommendations