Korean J Physiol Pharmacol.
2015 Jan;19(1):65-71. 10.4196/kjpp.2015.19.1.65.
Airway Smooth Muscle Sensitivity to Methacholine in Precision-Cut Lung Slices (PCLS) from Ovalbumin-induced Asthmatic Mice
- Affiliations
-
- 1Department of Physiology, Seoul National University College of Medicine, Seoul 110-799, Korea. physiolksj@gmail.com
- 2Ischemic/Hypoxic Disease Institute, Seoul National University College of Medicine, Seoul 110-799, Korea.
- 3Department of Internal Medicine, Seoul National University College of Medicine, Seoul 110-799, Korea.
- 4Chung-Ang University Red Cross College of Nursing, Seoul 100-031, Korea.
- 5hannelopathy Research Center (CRC), Dongguk University College of Medicine, Goyang 410-773, Korea.
- KMID: 2071821
- DOI: http://doi.org/10.4196/kjpp.2015.19.1.65
Abstract
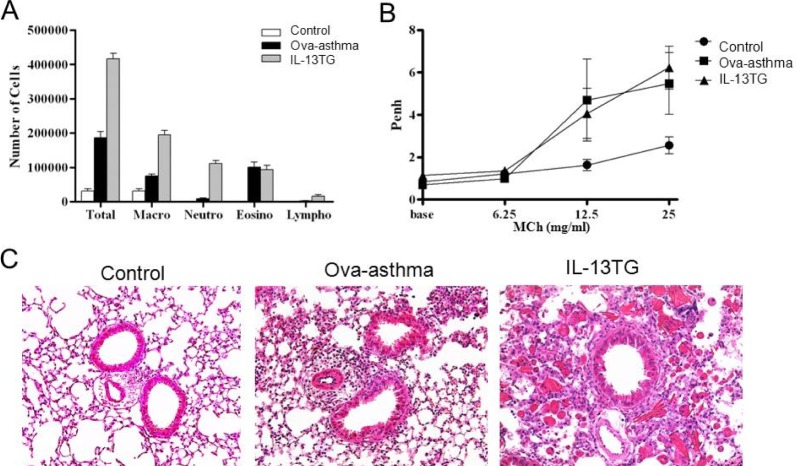
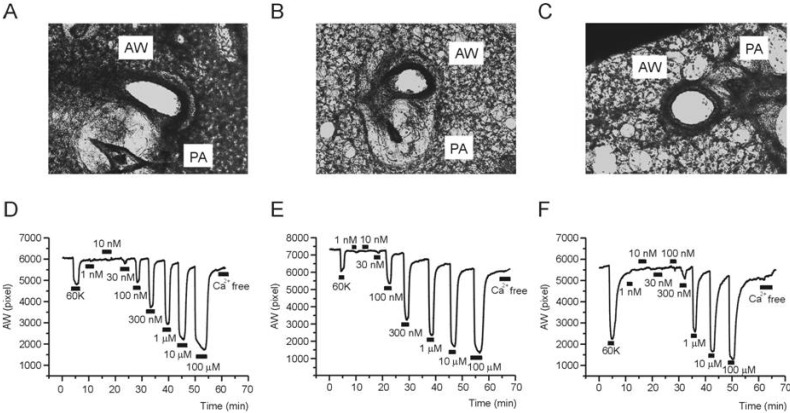
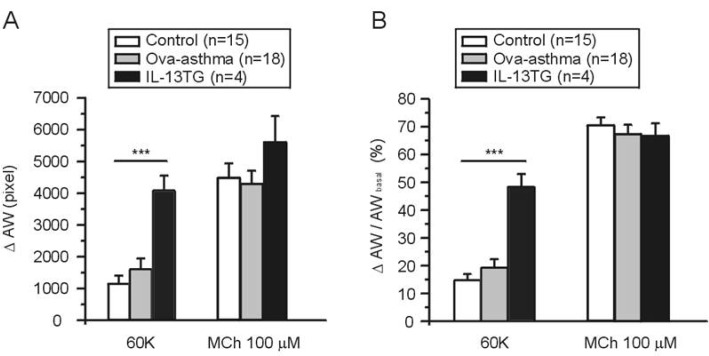
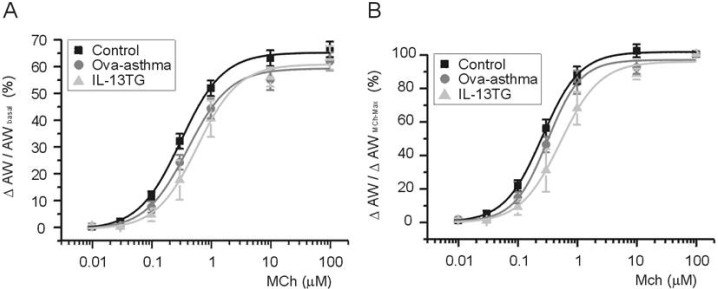
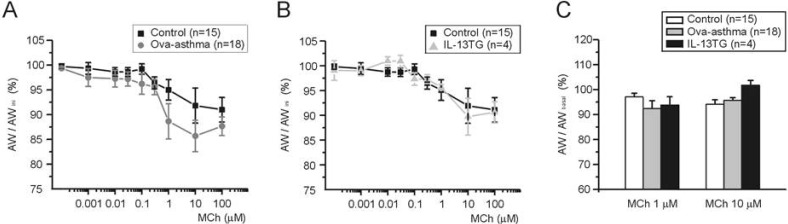
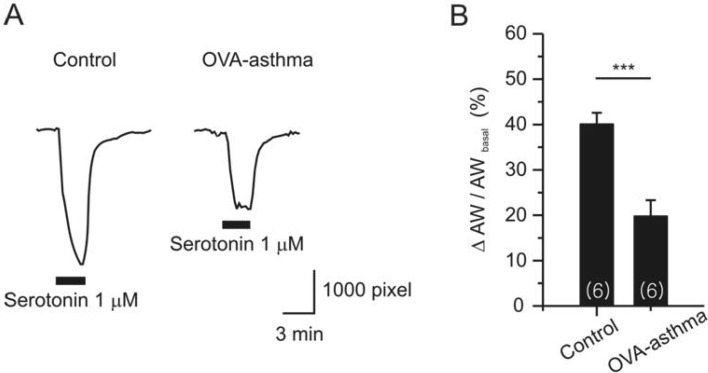
- Asthma is a chronic inflammatory disease characterized by airway hyperresponsiveness (AHR) and reversible airway obstruction. Methacholine (MCh) is widely used in broncho-provocation test to evaluate airway resistance. For experimental investigation, ovalbumin-induced sensitization is frequently used in rodents (Ova-asthma). However, albeit the inflammatory histology and AHR in vivo, it remains unclear whether the MCh sensitivity of airway smooth muscle isolated from Ova-asthma is persistently changed. In this study, the contractions of airways in precision-cut lung slices (PCLS) from control, Ova-asthma, and IL-13 overexpressed transgenic mice (IL-13TG) were compared by analyzing the airway lumen space (AW). The airway resistance in vivo was measured using plethysmograph. AHR and increased inflammatory cells in BAL fluid were confirmed in Ova-asthma and IL-13TG mice. In the PCLS from all three groups, MCh concentration-dependent narrowing of airway lumen (DeltaAW) was observed. In contrast to the AHR in vivo, the EC50 of MCh for DeltaAW from Ova-asthma and IL-13TG were not different from control, indicating unchanged sensitivity to MCh. Although the AW recovery upon MCh-washout showed sluggish tendency in Ova-asthma, the change was also statistically insignificant. Membrane depolarization-induced DeltaAW by 60 mM K+ (60K-contraction) was larger in IL-13TG than control, whereas 60K-contraction of Ova-asthma was unaffected. Furthermore, serotonin-induced DeltaAW of Ova-asthma was smaller than control and IL-13TG. Taken together, the AHR in Ova-asthma and IL-13TG are not reflected in the contractility of isolated airways from PCLS. The AHR of the model animals seems to require intrinsic agonists or inflammatory microenvironment that is washable during tissue preparation.
Keyword
MeSH Terms
Figure
Reference
-
1. Sumino K, Sugar EA, Irvin CG, Kaminsky DA, Shade D, Wei CY, Holbrook JT, Wise RA, Castro M. American Lung Association Asthma Clinical Research Centers. Methacholine challenge test: diagnostic characteristics in asthmatic patients receiving controller medications. J Allergy Clin Immunol. 2012; 130:69–75.e6. PMID: 22465214.2. Dixon C. The bronchial challenge test: a new direction in asthmatic management. J Natl Med Assoc. 1983; 75:199–204. PMID: 6827612.3. Wills-Karp M. Interleukin-13 in asthma pathogenesis. Immunol Rev. 2004; 202:175–190. PMID: 15546393.
Article4. Matsumoto H, Hirata Y, Otsuka K, Iwata T, Inazumi A, Niimi A, Ito I, Ogawa E, Muro S, Sakai H, Chin K, Oku Y, Mishima M. Interleukin-13 enhanced Ca2+ oscillations in airway smooth muscle cells. Cytokine. 2012; 57:19–24. PMID: 22078634.5. Kasaian MT, Miller DK. IL-13 as a therapeutic target for respiratory disease. Biochem Pharmacol. 2008; 76:147–155. PMID: 18502398.
Article6. Shin YS, Takeda K, Gelfand EW. Understanding asthma using animal models. Allergy Asthma Immunol Res. 2009; 1:10–18. PMID: 20224665.
Article7. Mullane K, Williams M. Animal models of asthma: reprise or reboot? Biochem Pharmacol. 2014; 87:131–139. PMID: 23831953.
Article8. Held HD, Martin C, Uhlig S. Characterization of airway and vascular responses in murine lungs. Br J Pharmacol. 1999; 126:1191–1199. PMID: 10205008.
Article9. Blacquière MJ, Hylkema MN, Postma DS, Geerlings M, Timens W, Melgert BN. Airway inflammation and remodeling in two mouse models of asthma: comparison of males and females. Int Arch Allergy Immunol. 2010; 153:173–181. PMID: 20413985.
Article10. Park SJ, Yoo HY, Kim HJ, Kim JK, Zhang YH, Kim SJ. Requirement of pretone by thromboxane A(2) for hypoxic pulmonary vasoconstriction in precision-cut lung slices of rat. Korean J Physiol Pharmacol. 2012; 16:59–64. PMID: 22416221.11. Martin C, Uhlig S, Ullrich V. Videomicroscopy of methacholine-induced contraction of individual airways in precision-cut lung slices. Eur Respir J. 1996; 9:2479–2487. PMID: 8980957.
Article12. Henjakovic M, Martin C, Hoymann HG, Sewald K, Ressmeyer AR, Dassow C, Pohlmann G, Krug N, Uhlig S, Braun A. Ex vivo lung function measurements in precision-cut lung slices (PCLS) from chemical allergen-sensitized mice represent a suitable alternative to in vivo studies. Toxicol Sci. 2008; 106:444–453. PMID: 18775882.
Article13. Chew AD, Hirota JA, Ellis R, Wattie J, Inman MD, Janssen LJ. Effects of allergen on airway narrowing dynamics as assessed by lung-slice technique. Eur Respir J. 2008; 31:532–538. PMID: 18032442.
Article14. Kim YK, Oh SY, Jeon SG, Park HW, Lee SY, Chun EY, Bang B, Lee HS, Oh MH, Kim YS, Kim JH, Gho YS, Cho SH, Min KU, Kim YY, Zhu Z. Airway exposure levels of lipopolysaccharide determine type 1 versus type 2 experimental asthma. J Immunol. 2007; 178:5375–5382. PMID: 17404323.
Article15. Hamelmann E, Schwarze J, Takeda K, Oshiba A, Larsen GL, Irvin CG, Gelfand EW. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am J Respir Crit Care Med. 1997; 156:766–775. PMID: 9309991.
Article16. Erle DJ, Sheppard D. The cell biology of asthma. J Cell Biol. 2014; 205:621–631. PMID: 24914235.
Article17. Gosling M, Poll C, Li S. TRP channels in airway smooth muscle as therapeutic targets. Naunyn Schmiedebergs Arch Pharmacol. 2005; 371:277–284. PMID: 15917982.
Article18. Gao YD, Zou JJ, Zheng JW, Shang M, Chen X, Geng S, Yang J. Promoting effects of IL-13 on Ca2+ release and store-operated Ca2+ entry in airway smooth muscle cells. Pulm Pharmacol Ther. 2010; 23:182–189. PMID: 20045483.19. Gao YD, Zheng JW, Li P, Cheng M, Yang J. Store-operated Ca2+ entry is involved in transforming growth factor-β1 facilitated proliferation of rat airway smooth muscle cells. J Asthma. 2013; 50:439–448. PMID: 23452113.20. Suganuma N, Ito S, Aso H, Kondo M, Sato M, Sokabe M, Hasegawa Y. STIM1 regulates platelet-derived growth factorinduced migration and Ca2+ influx in human airway smooth muscle cells. PLoS One. 2012; 7:e45056. PMID: 22984609.21. Ressmeyer AR, Larsson AK, Vollmer E, Dahlèn SE, Uhlig S, Martin C. Characterisation of guinea pig precision-cut lung slices: comparison with human tissues. Eur Respir J. 2006; 28:603–611. PMID: 16737991.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Requirement of Pretone by Thromboxane A2 for Hypoxic Pulmonary Vasoconstriction in Precision-cut Lung Slices of Rat
- Dermatophagoides Farinae-Induced Asthmatic Mouse Model of Airway Remodeling
- Effect of Dexamethasone on Airway Hyperresponsiveness and Airway Inflammation in Ovalbumin-induced Murine Asthma Model
- MicroRNA-21 inhibition attenuates airway inflammation and remodelling by modulating the transforming growth factor β–Smad7 pathway
- Effect of intranasal rosiglitazone on airway inflammation and remodeling in a murine model of chronic asthma