Korean J Physiol Pharmacol.
2012 Feb;16(1):59-64. 10.4196/kjpp.2012.16.1.59.
Requirement of Pretone by Thromboxane A2 for Hypoxic Pulmonary Vasoconstriction in Precision-cut Lung Slices of Rat
- Affiliations
-
- 1Department of Physiology, Seoul National University College of Medicine, Seoul 110-799, Korea. sjoonkim@snu.ac.kr
- 2Ischemic/Hypoxic Disease Institute, Seoul National University, Seoul 110-799, Korea.
- 3Kidney Institute Medical Research Center, Seoul National University, Seoul 110-799, Korea.
- 4Department of Anesthesiology and Pain Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul 135-710, Korea.
- KMID: 2285441
- DOI: http://doi.org/10.4196/kjpp.2012.16.1.59
Abstract
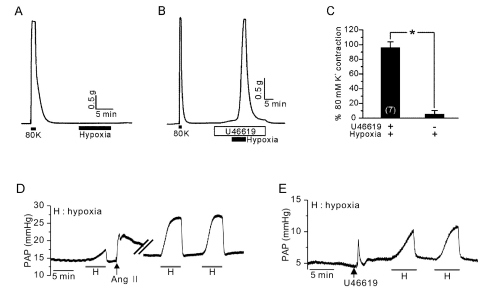
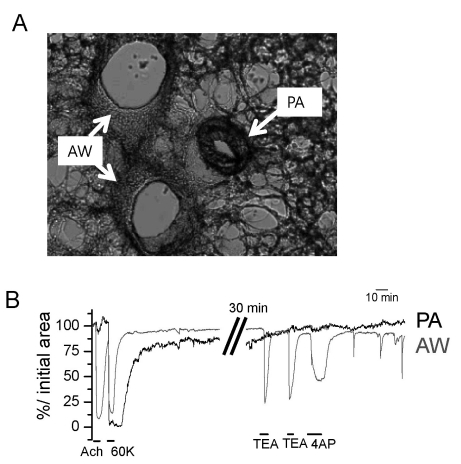
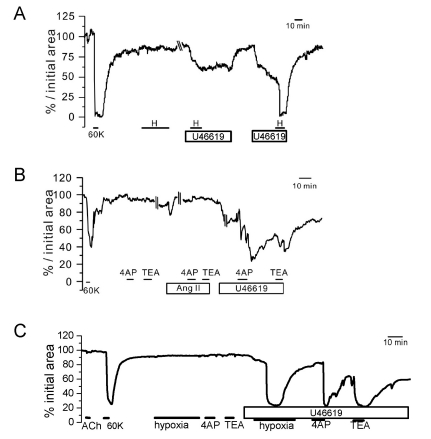
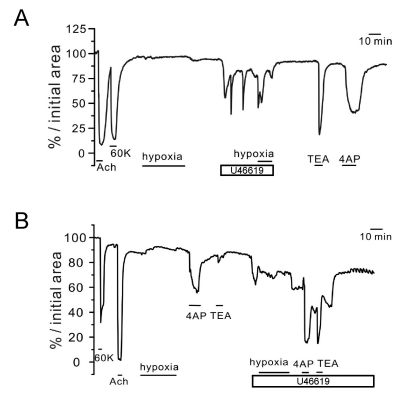
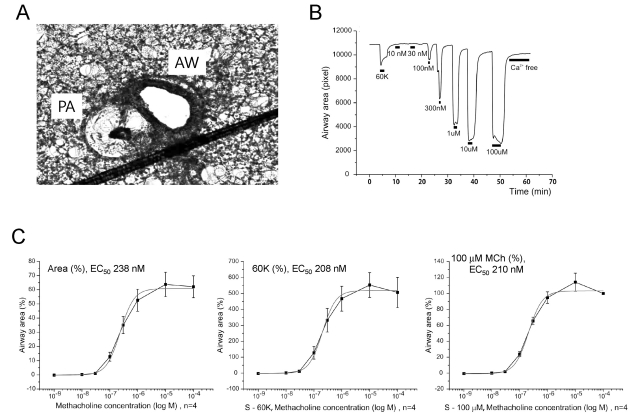
- Hypoxic pulmonary vasoconstriction (HPV) is physiologically important response for preventing mismatching between ventilation and perfusion in lungs. The HPV of isolated pulmonary arteries (HPV-PA) usually require a partial pretone by thromboxane agonist (U46619). Because the HPV of ventilated/perfused lungs (HPV-lung) can be triggered without pretone conditioning, we suspected that a putative tissue factor might be responsible for the pretone of HPV. Here we investigated whether HPV can be also observed in precision-cut lung slices (PCLS) from rats. The HPV in PCLS also required partial contraction by U46619. In addition, K+ channel blockers (4AP and TEA) required U46619-pretone to induce significant contraction of PA in PCLS. In contrast, the airways in PCLS showed reversible contraction in response to the K+ channel blockers without pretone conditioning. Also, the airways showed no hypoxic constriction but a relaxation under the partial pretone by U46619. The airways in PCLS showed reliable, concentration-dependent contraction by metacholine (EC50, ~210 nM). In summary, the HPV in PCLS is more similar to isolated PA than V/P lungs. The metacholine-induced constriction of bronchioles suggested that the PLCS might be also useful for studying airway physiology in situ.
MeSH Terms
Figure
Reference
-
1. Bonnet S, Dubuis E, Vandier C, Martin S, Marthan R, Savineau JP. Reversal of chronic hypoxia-induced alterations in pulmonary artery smooth muscle electromechanical coupling upon air breathing. Cardiovasc Res. 2002; 53:1019–1028. PMID: 11922912.
Article2. Osipenko ON, Alexander D, MacLean MR, Gurney AM. Influence of chronic hypoxia on the contributions of non-inactivating and delayed rectifier K currents to the resting potential and tone of rat pulmonary artery smooth muscle. Br J Pharmacol. 1998; 124:1335–1337. PMID: 9723941.
Article3. Yoo HY, Park SJ, Seo EY, Park KS, Han JA, Kim KS, Shin DH, Earm YE, Zhang YH, Kim SJ. Role of thromboxane A2-activated nonselective cation channels in hypoxic pulmonary vasoconstriction of rat. Am J Physiol Cell Physiol. 2012; 302:C307–C317. PMID: 21998141.
Article4. Leach RM, Robertson TP, Twort CH, Ward JP. Hypoxic vasoconstriction in rat pulmonary and mesenteric arteries. Am J Physiol. 1994; 266:L223–L231. PMID: 8166292.
Article5. Rodman DM, Yamaguchi T, O'Brien RF, McMurtry IF. Hypoxic contraction of isolated rat pulmonary artery. J Pharmacol Exp Ther. 1989; 248:952–959. PMID: 2467984.6. Park SJ, Yoo HY, Earm YE, Kim SJ, Kim JK, Kim SD. Role of arachidonic acid-derived metabolites in the control of pulmonary arterial pressure and hypoxic pulmonary vasoconstriction in rats. Br J Anaesth. 2011; 106:31–37. PMID: 20935003.
Article7. Sanderson MJ. Exploring lung physiology in health and disease with lung slices. Pulm Pharmacol Ther. 2011; 24:452–465. PMID: 21600999.
Article8. Struckmann N, Schwering S, Wiegand S, Gschnell A, Yamada M, Kummer W, Wess J, Haberberger RV. Role of muscarinic receptor subtypes in the constriction of peripheral airways: studies on receptor-deficient mice. Mol Pharmacol. 2003; 64:1444–1451. PMID: 14645675.
Article9. Moreno L, Perez-Vizcaino F, Harrington L, Faro R, Sturton G, Barnes PJ, Mitchell JA. Pharmacology of airways and vessels in lung slices in situ: role of endogenous dilator hormones. Respir Res. 2006; 7:111. PMID: 16923180.
Article10. Paddenberg R, König P, Faulhammer P, Goldenberg A, Pfeil U, Kummer W. Hypoxic vasoconstriction of partial muscular intra-acinar pulmonary arteries in murine precision cut lung slices. Respir Res. 2006; 7:93. PMID: 16808843.
Article11. Bai Y, Sanderson MJ. Modulation of the Ca2+ sensitivity of airway smooth muscle cells in murine lung slices. Am J Physiol Lung Cell Mol Physiol. 2006; 291:L208–L221. PMID: 16461427.12. Martin C, Uhlig S, Ullrich V. Cytokine-induced bronchoconstriction in precision-cut lung slices is dependent upon cyclooxygenase-2 and thromboxane receptor activation. Am J Respir Cell Mol Biol. 2001; 24:139–145. PMID: 11159047.
Article13. Bergner A, Sanderson MJ. Acetylcholine-induced calcium signaling and contraction of airway smooth muscle cells in lung slices. J Gen Physiol. 2002; 119:187–198. PMID: 11815668.
Article14. Brueggemann LI, Kakad PP, Love RB, Solway J, Dowell ML, Cribbs LL, Byron KL. Kv7 potassium channels in airway smooth muscle cells: signal transduction intermediates and pharmacological targets for bronchodilator therapy. Am J Physiol Lung Cell Mol Physiol. 2012; 302:L120–L132. PMID: 21964407.
Article15. Ressmeyer AR, Larsson AK, Vollmer E, Dahlèn SE, Uhlig S, Martin C. Characterisation of guinea pig precision-cut lung slices: comparison with human tissues. Eur Respir J. 2006; 28:603–611. PMID: 16737991.
Article16. Desireddi JR, Farrow KN, Marks JD, Waypa GB, Schumacker PT. Hypoxia increases ROS signaling and cytosolic Ca2+ in pulmonary artery smooth muscle cells of mouse lungs slices. Antioxid Redox Signal. 2010; 12:595–602. PMID: 19747064.17. Held HD, Martin C, Uhlig S. Characterization of airway and vascular responses in murine lungs. Br J Pharmacol. 1999; 126:1191–1199. PMID: 10205008.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Disappearance of Hypoxic Pulmonary Vasoconstriction and O2-Sensitive Nonselective Cationic Current in Arterial Myocytes of Rats Under Ambient Hypoxia
- The Effect of Isoproterenol, Dobutamine, and Milrinone on Pulmonary Vasoconstriction in Isolated Rat Lungs
- Effects of Glibenclamide and L-NAME on Hypoxic Pulmonary Vasoconstriction in Rats
- Pulmonary Hypertension Associated with Acute Hypoxic Pulmonary Vasoconstriction in a Patient with Acute Myeloid Leukemia
- PGF2 alpha causes bronchoconstriction and pulmonary vasoconstriction via thromboxane receptors in rat lung