Korean J Physiol Pharmacol.
2011 Oct;15(5):279-283. 10.4196/kjpp.2011.15.5.279.
Quercetin-induced Growth Inhibition in Human Bladder Cancer Cells Is Associated with an Increase in Ca(2+)-activated K+ Channels
- Affiliations
-
- 1Department of Physiology, Chungbuk National University, Cheongju 361-763, Korea. yangmik@chungbuk.ac.kr
- 2Department of Urology, Chungbuk National University, Cheongju 361-763, Korea.
- 3Department of Biomedical Engineering, Chungbuk National University, Cheongju 361-763, Korea.
- 4Personalized Tumor Engineering Research Center, College of Medicine, Chungbuk National University, Cheongju 361-763, Korea.
- KMID: 2071730
- DOI: http://doi.org/10.4196/kjpp.2011.15.5.279
Abstract
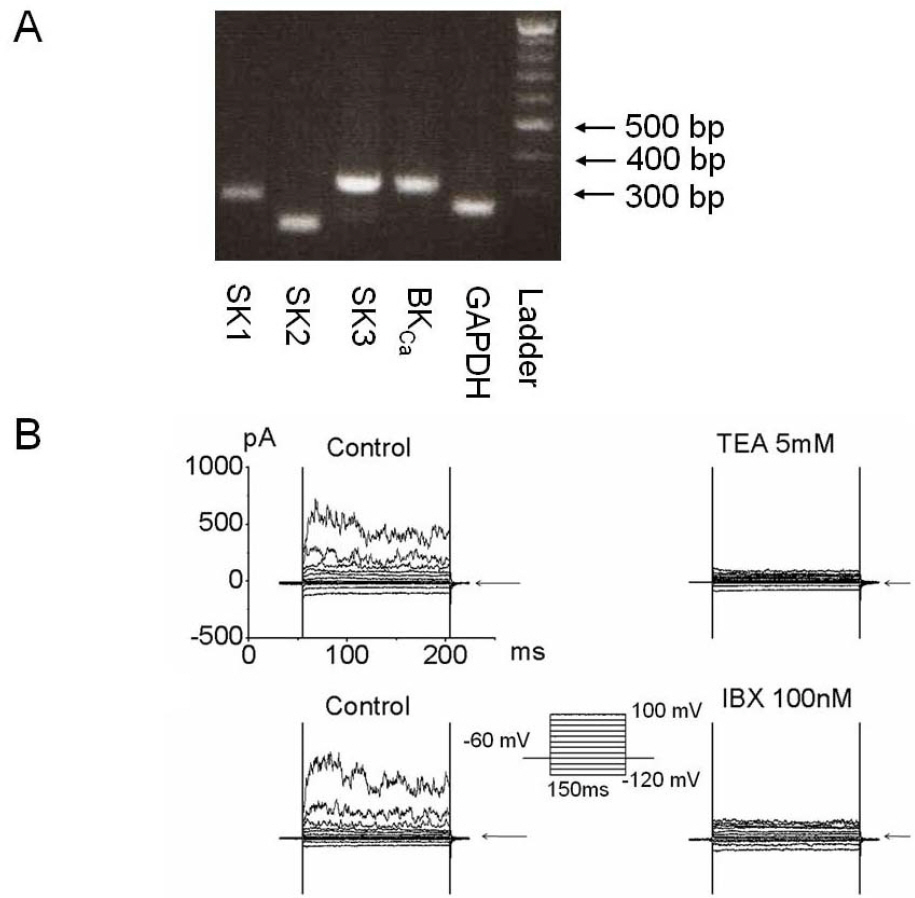
- Quercetin (3,3',4',5,7-pentahydroxyflavone) is an attractive therapeutic flavonoid for cancer treatment because of its beneficial properties including apoptotic, antioxidant, and antiproliferative effects on cancer cells. However, the exact mechanism of action of quercetin on ion channel modulation is poorly understood in bladder cancer 253J cells. In this study, we demonstrated that large conductance Ca2+-activated K+ (BKCa) or MaxiK channels were functionally expressed in 253J cells, and quercetin increased BKCa current in a concentration dependent and reversible manner using a whole cell patch configuration. The half maximal activation concentration (IC50) of quercetin was 45.5+/-7.2 microM. The quercetin-evoked BKCa current was inhibited by tetraethylammonium (TEA; 5 mM) a non-specific BKCa blocker and iberiotoxin (IBX; 100 nM) a BKCa-specific blocker. Quercetin-induced membrane hyperpolarization was measured by fluorescence-activated cell sorting (FACS) with voltage sensitive dye, bis (1,3-dibutylbarbituric acid) trimethine oxonol (DiBAC4(3); 100 nM). Quercetin-evoked hyperpolarization was prevented by TEA. Quercetin produced an antiproliferative effect (30.3+/-13.5%) which was recovered to 53.3+/-10.5% and 72.9+/-3.7% by TEA and IBX, respectively. Taken together our results indicate that activation of BKCa channels may be considered an important target related to the action of quercetin on human bladder cancer cells.
MeSH Terms
Figure
Reference
-
References
1. Hirpara KV, Aggarwal P, Mukherjee AJ, Joshi N, Burman AC. Quercetin and its derivatives: synthesis, pharmacological uses with special emphasis on anti-tumor properties and prodrug with enhanced bio-availability. Anticancer Agents Med Chem. 2009; 9:138–161.
Article2. Okamoto T. Safety of quercetin for clinical application (Review). Int J Mol Med. 2005; 16:275–278.
Article3. Ruiz MJ, Fernández M, Picó Y, Mañes J, Asensi M, Carda C, Asensio G, Estrela JM. Dietary administration of high doses of pterostilbene and quercetin to mice is not toxic. J Agric Food Chem. 2009; 57:3180–3186.
Article4. Avila MA, Velasco JA, Cansado J, Notario V. Quercetin mediates the down-regulation of mutant p53 in the human breast cancer cell line MDA-MB468. Cancer Res. 1994; 54:2424–2428.5. Kim HJ, Kim SK, Kim BS, Lee SH, Park YS, Park BK, Kim SJ, Kim J, Choi C, Kim JS, Cho SD, Jung JW, Roh KH, Kang KS, Jung JY. Apoptotic effect of quercetin on HT-29 colon cancer cells via the AMPK signaling pathway. J Agric Food Chem. 2010; 58:8643–8650.
Article6. Wang HY, Guo LM, Chen Y, Zhao XH, Cheng CL, Wu MY, He LY. Quercetin inhibits growth and induces apoptosis of human gastric carcinoma cells. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2006; 22:585–587.7. Monasterio A, Urdaci MC, Pinchuk IV, López-Moratalla N, Martínez-Irujo JJ. Flavonoids induce apoptosis in human leukemia U937 cells through caspase- and caspase-calpain-dependent pathways. Nutr Cancer. 2004; 50:90–100.
Article8. Ma L, Feugang JM, Konarski P, Wang J, Lu J, Fu S, Ma B, Tian B, Zou C, Wang Z. Growth inhibitory effects of quercetin on bladder cancer cell. Front Biosci. 2006; 11:2275–2285.
Article9. Zhang M, Swarts SG, Yin L, Liu C, Tian Y, Cao Y, Swarts M, Yang S, Zhang SB, Zhang K, Ju S, Olek DJ Jr, Schwartz L, Keng PC, Howell R, Zhang L, Okunieff P. Antioxidant properties of quercetin. Adv Exp Med Biol. 2011; 701:283–289.
Article10. Moon SK, Cho GO, Jung SY, Gal SW, Kwon TK, Lee YC, Madamanchi NR, Kim CH. Quercetin exerts multiple inhibitory effects on vascular smooth muscle cells: role of ERK1/2, cell-cycle regulation, and matrix metalloproteinase-9. Biochem Biophys Res Commun. 2003; 301:1069–78.
Article11. Choi JA, Kim JY, Lee JY, Kang CM, Kwon HJ, Yoo YD, Kim TW, Lee YS, Lee SJ. Induction of cell cycle arrest and apoptosis in human breast cancer cells by quercetin. Int J Oncol. 2001; 19:837–844.
Article12. Beniston RG, Campo MS. Quercetin elevates p27(Kip1) and arrests both primary and HPV16 E6/E7 transformed human keratinocytes in G1. Oncogene. 2003; 22:5504–5514.13. Mittra B, Saha A, Chowdhury AR, Pal C, Mandal S, Mukhopadhyay S, Bandyopadhyay S, Majumder HK. Luteolin, an abundant dietary component is a potent anti-leishmanial agent that acts by inducing topoisomerase II-mediated kinetoplast DNA cleavage leading to apoptosis. Mol Med. 2000; 6:527–541.
Article14. Vidya Priyadarsini R, Senthil Murugan R, Maitreyi S, Ramalingam K, Karunagaran D, Nagini S. The flavonoid quercetin induces cell cycle arrest and mitochondria-mediated apoptosis in human cervical cancer (HeLa) cells through p53 induction and NF-κB inhibition. Eur J Pharmacol. 2010; 649:84–91.
Article15. Wang Z. Roles of K+ channels in regulating tumour cell proliferation and apoptosis. Pflugers Arch. 2004; 448:274–286.16. Saponara S, Sgaragli G, Fusi F. Quercetin as a novel activator of L-type Ca(2+) channels in rat tail artery smooth muscle cells. Br J Pharmacol. 2002; 135:1819–1827.17. Saponara S, Sgaragli G, Fusi F. Quercetin antagonism of Bay K 8644 effects on rat tail artery L-type Ca2+ channels. Eur J Pharmacol. 2008; 598:75–80.18. Nishida S, Satoh H. Possible Involvement of Ca activated K channels, SK channel, in the quercetin-induced vasodilatation. Korean J Physiol Pharmacol. 2009; 13:361–365.19. Kuhlmann CR, Schaefer CA, Kosok C, Abdallah Y, Walther S, Lüdders DW, Neumann T, Tillmanns H, Schäfer C, Piper HM, Erdogan A. Quercetin-induced induction of the NO/cGMP pathway depends on Ca2+-activated K+ channel-induced hyper-polarization-mediated Ca2+-entry into cultured human endothelial cells. Planta Med. 2005; 71:520–524.20. Yang L, Ma JH, Zhang PH, Zou AR, Tu DN. Quercetin activates human Kv1.5 channels by a residue I502 in the S6 segment. Clin Exp Pharmacol Physiol. 2009; 36:154–161.
Article21. Pyle LC, Fulton JC, Sloane PA, Backer K, Mazur M, Prasain J, Barnes S, Clancy JP, Rowe SM. Activation of the cystic fibrosis transmembrane conductance regulator by the flavonoid quercetin: potential use as a biomarker of ΔF508 cystic fibrosis transmembrane conductance regulator rescue. Am J Respir Cell Mol Biol. 2010; 43:607–616.22. Lee BH, Hwang SH, Choi SH, Shin TJ, Kang J, Lee SM, Nah SY. Quercetin Inhibits α3β4 Nicotinic acetylcholine receptor-mediated ion currents expressed in Xenopus oocytes. Korean J Physiol Pharmacol. 2011; 15:17–22.23. Ouadid-Ahidouch H, Roudbaraki M, Ahidouch A, Delcourt P, Prevarskaya N. Cell-cycle-dependent expression of the large Ca2+-activated K+ channels in breast cancer cells. Biochem Biophys Res Commun. 2004; 316:244–251.24. Han X, Xi L, Wang H, Huang X, Ma X, Han Z, Wu P, Ma X, Lu Y, Wang G, Zhou J, Ma D. The potassium ion channel opener NS1619 inhibits proliferation and induces apoptosis in A2780 ovarian cancer cells. Biochem Biophys Res Commun. 2008; 375:205–209.
Article25. Cambien B, Rezzonico R, Vitale S, Rouzaire-Dubois B, Dubois JM, Barthel R, Karimdjee BS, Mograbi B, Schmid-Alliana A, Schmid-Antomarchi H. Silencing of hSlo potassium channels in human osteosarcoma cells promotes tumorigenesis. Int J Cancer. 2008; 123:365–371.
Article26. Abdullaev IF, Rudkouskaya A, Mongin AA, Kuo YH. Calcium-activated potassium channels BK and IK1 are functionally expressed in human gliomas but do not regulate cell proliferation. PLoS One. 2010; 5:e12304.
Article27. Roger S, Potier M, Vandier C, Le Guennec JY, Besson P. Description and role in proliferation of iberiotoxin-sensitive currents in different human mammary epithelial normal and cancerous cells. Biochim Biophys Acta. 2004; 1667:190–199.
Article28. Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981; 391:85–100.
Article29. Yamada A, Gaja N, Ohya S, Muraki K, Narita H, Ohwada T, Imaizumi Y. Usefulness and limitation of DiBAC4(3), a voltage-sensitive fluorescent dye, for the measurement of membrane potentials regulated by recombinant large conductance Ca2+-activated K+ channels in HEK293 cells. Jpn J Pharmacol. 2001; 86:342–350.30. Lang F, Föller M, Lang K, Lang P, Ritter M, Vereninov A, Szabo I, Huber SM, Gulbins E. Cell volume regulatory ion channels in cell proliferation and cell death. Methods Enzymol. 2007; 428:209–225.
Article31. Huang MH, Wu SN, Chen CP, Shen AY. Inhibition of Ca2+-activated and voltage-dependent K+ currents by 2-mercaptophenyl-1,4-naphthoquinone in pituitary GH3 cells: contribution to its antiproliferative effect. Life Sci. 2002; 70:1185–1203.32. Ransom CB, Sontheimer H. BK channels in human glioma cells. J Neurophysiol. 2001; 85:790–803.
Article33. Basrai D, Kraft R, Bollensdorff C, Liebmann L, Benndorf K, Patt S. BK channel blockers inhibit potassium-induced proliferation of human astrocytoma cells. Neuroreport. 2002; 13:403–407.
Article34. Weaver AK, Bomben VC, Sontheimer H. Expression and function of calcium-activated potassium channels in human glioma cells. Glia. 2006; 54:223–233.
Article35. Coiret G, Borowiec AS, Mariot P, Ouadid-Ahidouch H, Matifat F. The antiestrogen tamoxifen activates BK channels and stimulates proliferation of MCF-7 breast cancer cells. Mol Pharmacol. 2007; 71:843–851.
Article36. Bloch M, Ousingsawat J, Simon R, Schraml P, Gasser TC, Mihatsch MJ, Kunzelmann K, Bubendorf L. KCNMA1 gene amplification promotes tumor cell proliferation in human prostate cancer. Oncogene. 2007; 26:2525–2534.
Article37. Kakehi Y, Hirao Y, Kim WJ, Ozono S, Masumori N, Miyanaga N, Nasu Y, Yokomizo A. Bladder Cancer Working Group report. Jpn J Clin Oncol. 2010; 40 Suppl. 1:i57–64.
Article38. Shang PF, Kwong J, Wang ZP, Tian J, Jiang L, Yang K, Yue ZJ, Tian JQ. Intravesical Bacillus Calmette-Guérin versus epirubicin for Ta and T1 bladder cancer. Cochrane Database Syst Rev. 2011. (5):CD006885.
Article39. Friberg S. BCG in the treatment of superficial cancer of the bladder: a review. Med Oncol Tumor Pharmacother. 1993; 10:31–36.
Article40. Lockyer CR, Gillatt DA. BCG immunotherapy for superficial bladder cancer. J R Soc Med. 2001; 94:119–123.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Quercetin on the BK(Ca) in Umbilical Cord Vein-derived Mesenchymal Stem Cells
- Quercetin induces cell death by caspase-dependent and p38 MAPK pathway in EGFR mutant lung cancer cells
- Autophagy Inhibition Promotes Quercetin Induced Apoptosis in MG-63 Human Osteosarcoma cells
- Possible Involvement of Ca2+ Activated K+ Channels, SK Channel, in the Quercetin-Induced Vasodilatation
- Quercetin-induced apoptosis ameliorates vascular smooth muscle cell senescence through AMP-activated protein kinase signaling pathway