Korean J Physiol Pharmacol.
2009 Aug;13(4):321-326. 10.4196/kjpp.2009.13.4.321.
Antioxidant Effect of CoQ10 on N-nitrosodiethylamine-induced Oxidative Stress in Mice
- Affiliations
-
- 1Department of Pathophysiology, College of Pharmacy, Chung-Ang University, Seoul 156-756, Korea. simss@cau.ac.kr
- 2Department of Pharmacology, College of Pharmacy, Chung-Ang University, Seoul 156-756, Korea.
- KMID: 2071688
- DOI: http://doi.org/10.4196/kjpp.2009.13.4.321
Abstract
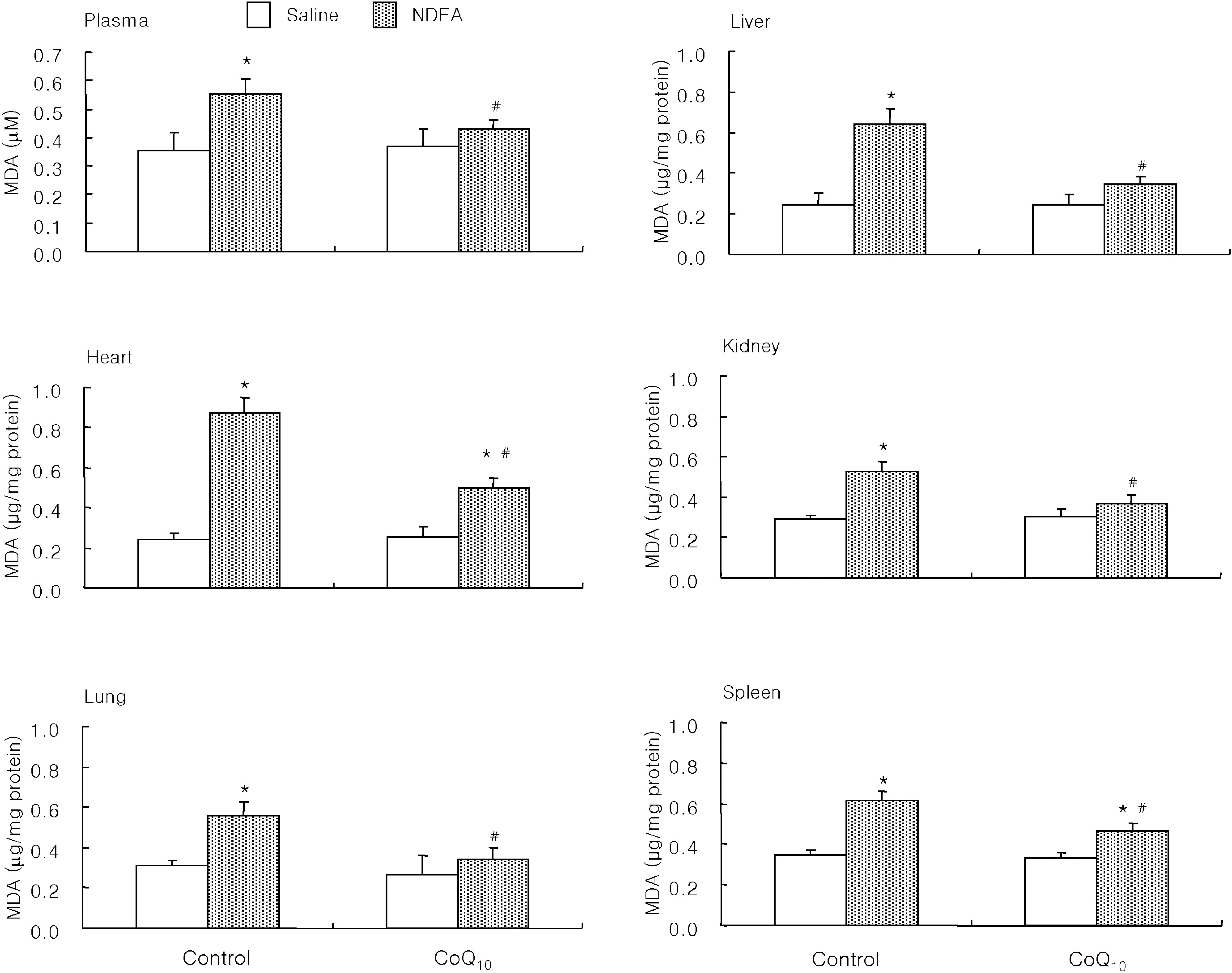
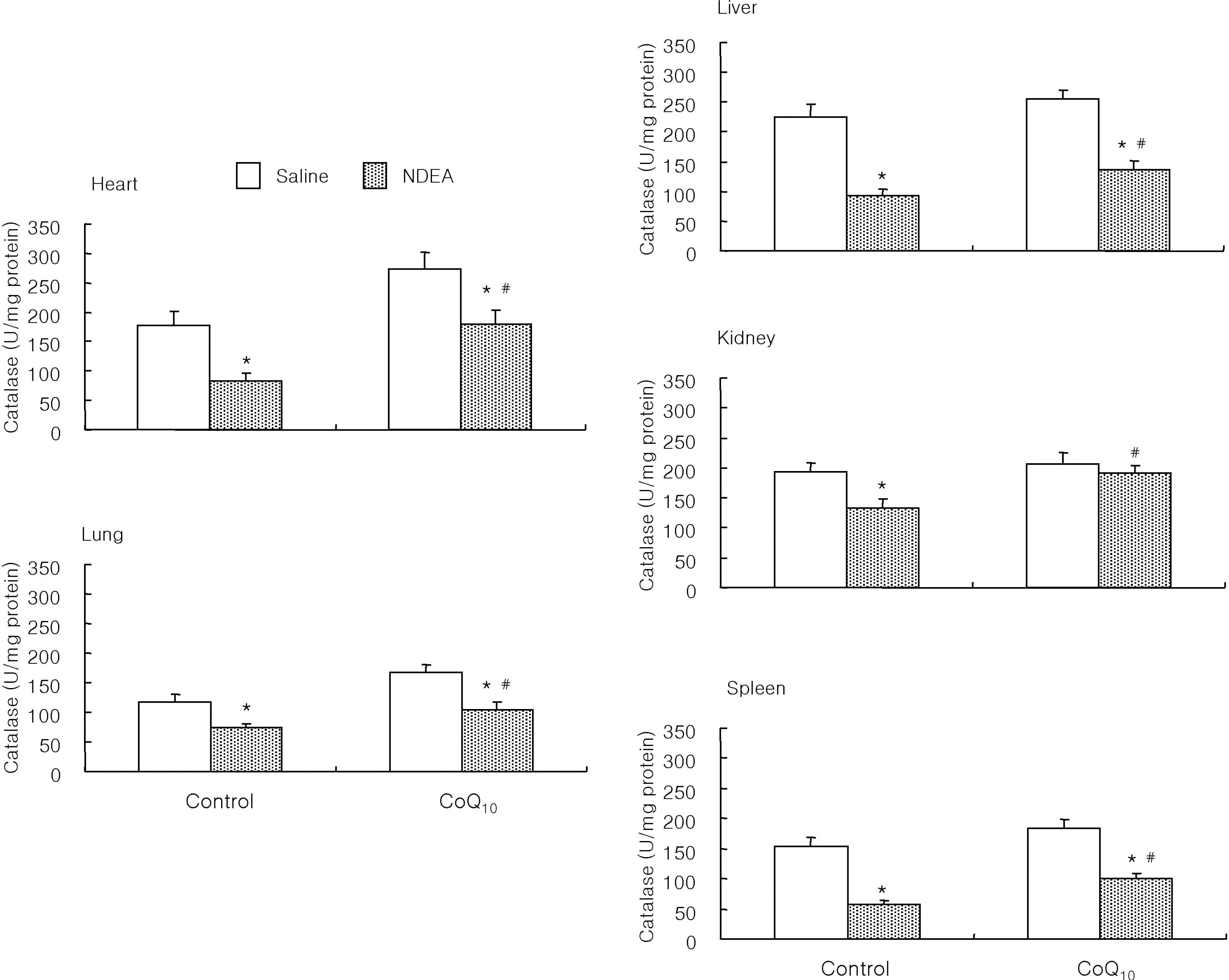
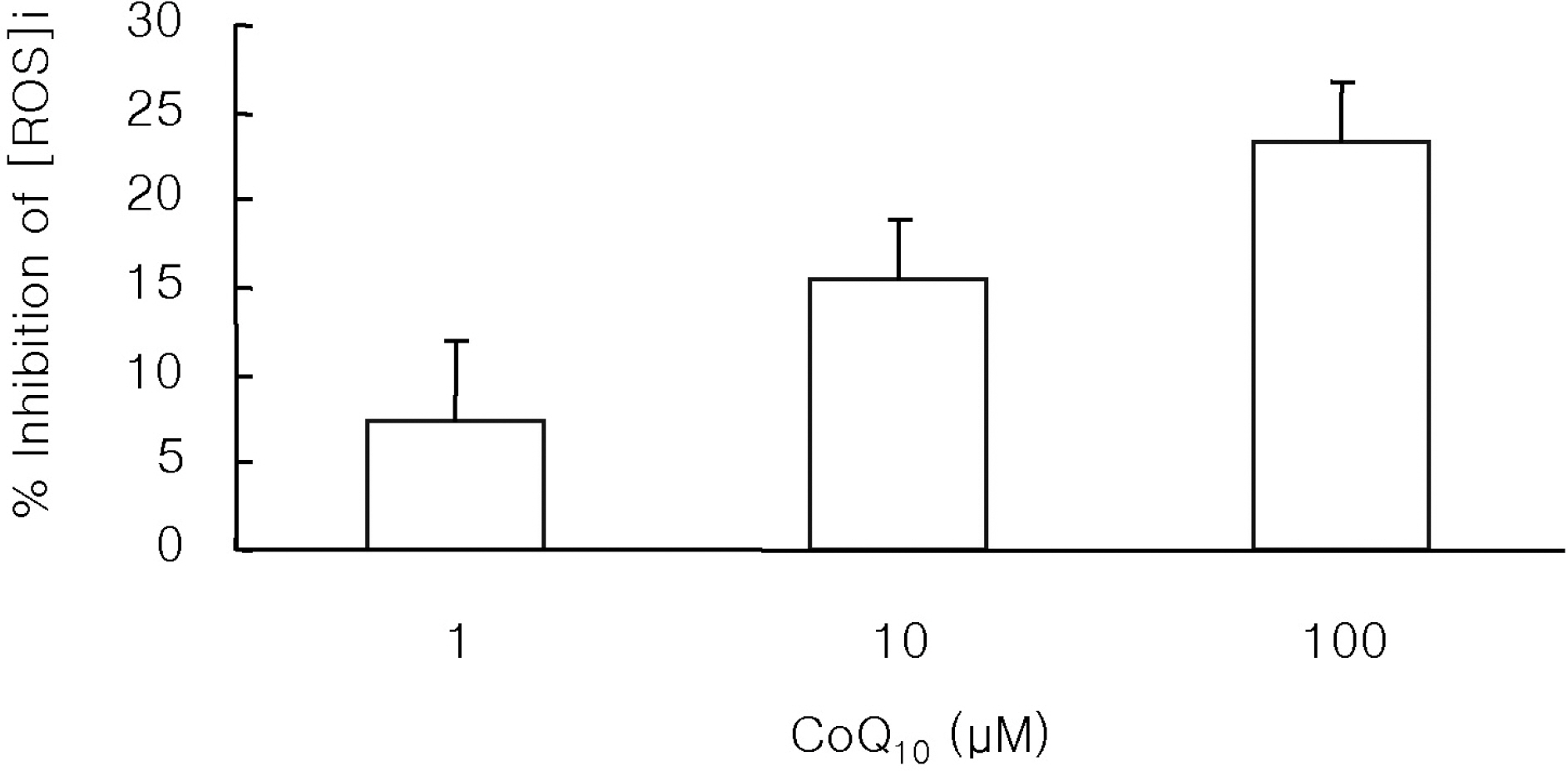
- The antioxidant effect of CoQ10 on N-nitrosodiethylamine (NDEA)-induced oxidative stress was investigated in mice. Food intake and body weight were similar in both CoQ10 and control groups during the 3-week experimental period. NDEA significantly increased the activities of typical marker enzymes of liver function (AST, ALT and ALP) both in control and CoQ10 groups. However, the increase of plasma aminotransferase activity was significantly reduced in the CoQ10 group. Lipid peroxidation in various tissues, such as heart, lung, liver, kidney, spleen and plasma, was significantly increased by NDEA, but this increase was significantly reduced by 100 mg/kg of CoQ10. Superoxide dismutase activity increased significantly upon NDEA-induced oxidative stress in both the control and CoQ10 groups with the effect being less in the CoQ10 group. Catalase activity decreased significantly in both the control and CoQ10 groups treated with NDEA, again with the effect being less in the CoQ10 group. The lesser effect on superoxide dismutase and catalase in the NDEA-treated CoQ10 group is indicative of the protective effect CoQ10. Thus, CoQ10 can offer useful protection against NDEA-induced oxidative stress.
MeSH Terms
Figure
Reference
-
Ames BN., Shigenga MK., Hagen TM. Oxidants, antioxidants and the degenerative diseases of aging. Proc Natl Acad Sci. 90:7915–7922. 1993.
ArticleBansal AK., Bansal M., Soni G., Bhatnagar D. Modulation of NDEA induced oxidative stress by vitamin E in rat erythrocytes. Hum Exp Toxicol. 24:297–302. 2005.Bansal AK., Bhatnagar D., Soni GL. In vitro effect of N-nitrosodi-methylamine on lipid peroxidation and antioxidant system in human erythrocytes. Toxicol In Vitro. 10:649–653. 1996.Bartsch H., Hietanen E., Malaveille C. Carcinogenic nitrosamines: free radical aspects of their action. Free Radic Biol Med. 7:637–644. 1989.
ArticleBeckman KB., Ames BN. The free radical theory of aging matures. Physiol Rev. 78:547–581. 1998.
ArticleBoland A., Delapierre D., Mossay D., Hans P., Dresse A. Propofol protects cultured brain cells from iron ion-induced death: comparison with trolox. Eur J Pharmacol. 404:21–27. 2000.
ArticleCrane FL. Biochemical functions of coenzyme Q10. J Am Coll Nutr. 20:591–598. 2001.
ArticleErnster L., Dallner G. Biochemical, physiological and medical aspects of ubiquinone function. Biochim Biophys Acta. 1271:195–204. 1995.
ArticleKing EJ., Delory GE. The rates of enzyme hydrolysis of phosphoric esters. Biochem J. 33:11–85. 1959.Kirby AJ., Schmidt RJ. The antioxidant activity of Chinese herbs for eczema and of placebo herbs-I. J Ethnopharmacol. 56:103–108. 1997.Kohen R., Nyska A. Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions and methods for their quantification. Toxicol Pathol. 30:620–650. 2002.Marklund S., Marklund G. Involvement of the superoxide anion radical in the auto-oxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 47:469–474. 1974.McCune LM., Johns T. Antioxidant activity in medicinal plants associated with the symptoms of diabetes mellitus used by the indigenous peoples of the north American boreal forest. J Ethnopharmacol. 82:197–205. 2002.
ArticleNoguchi N., Watanabe A., Shi H. Diverse functions of antioxidants. Free Radic Res. 33:809–817. 2000.
ArticleNohl H., Gille L., Staniek K. The biochemical, pathophysiological, and medical aspects of ubiquinone function. Ann NY Acad Sci. 854:394–409. 1998.
ArticleOhkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric reaction. Anal Biochem. 95:351–358. 1979.Reitman S., Frenkel SA. Colorimetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvate transaminases. Am J Pathol. 28:56–63. 1957.Sawicka E., Dlugosz A. Toluene and P-xylene mixture exerts antagonistic effect on lipid peroxidation. in vitro. Int J Occup Med Environ Health. 21:201–209. 2008.
ArticleSena CM., Nunes E., Gomes A., Santos MS., Proença T., Martins MI., Seiça RM. Supplementation of coenzyme Q10 and alpha-tocopherol lowers glycated hemoglobin level and lipid peroxidation in pancreas of diabetic rats. Nutr Res. 28:113–121. 2008.Smith PK., Krohn RI., Hermanson GT., Mallia AK., Gartner FH., Provenzano MD., Fujimoto EK., Goeke NM., Olson BJ., Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 163:279–282. 1987.
ArticleSohal RS., Forster MJ. Coenzyme Q, oxidative stress and aging. Mitochondrion. 7S:S103–S111. 2007.
ArticleSpiteller G. Enzymic lipid peroxidation – a consequence of cell injury? Free Radic Biol Med. 21:1003–1009. 1996.Sumioka I., Matsura T., Kasuga S., Itakura Y., Yamada K. Mechanisms of protection by S-allylmercaptocysteine against acetaminophen-induced liver injury in mice. Jpn J Pharmacol. 78:199–207. 1998.Taniguchi M., Yasutake A., Takedomi K., Inoue K. Effects of N-nitrosodimethylamine (NDMA) on the oxidative status of rat liver. Arch Toxicol. 73:141–146. 1999.
ArticleThomas WS. The physiology and pharmacology of singlet oxygen. Med Hypothesis. 60:567–572. 2003.Woo CH., Eom YW., Yoo MH., You HJ., Han HJ., Song WK., Yoo YJ., Chun JS., Kim JH. Tumor necrosis factor-alpha generates reactive oxygen species via a cytosolic phospholipase A2-linked cascade. J Biol Chem. 275:32357–32362. 2000.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Coenzyme Q10 improves sperm motility and antioxidant status in infertile men with idiopathic oligoasthenospermia
- New Evidences of Neurotoxicity of Aroclor 1254 in Mice Brain: Potential of Coenzyme Q10 in Abating the Detrimental Outcomes
- Coenzyme Q10, oxidative stress markers, and sperm DNA damage in men with idiopathic oligoasthenoteratospermia
- Comparison of the effects of coenzyme Q10 and Centrum multivitamins on semen parameters, oxidative stress markers, and sperm DNA fragmentation in infertile men with idiopathic oligoasthenospermia
- Coenzyme Q10 Improves Sperm Parameters, Oxidative Stress Markers and Sperm DNA Fragmentation in Infertile Patients with Idiopathic Oligoasthenozoospermia