Anat Cell Biol.
2012 Sep;45(3):160-169. 10.5115/acb.2012.45.3.160.
Expression of Bis in the mouse gastrointestinal system
- Affiliations
-
- 1Department of Biochemistry, The Catholic University of Korea College of Medicine, Seoul, Korea. leejh@catholic.ac.kr
- 2Department of Internal Medicine, The Catholic University of Korea College of Medicine, Seoul, Korea.
- 3Department of Anatomy, The Catholic University of Korea College of Medicine, Seoul, Korea.
- KMID: 2046743
- DOI: http://doi.org/10.5115/acb.2012.45.3.160
Abstract
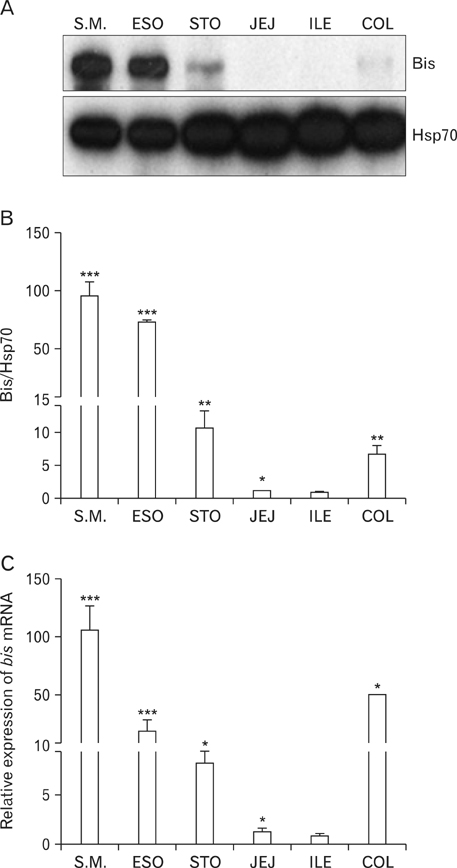
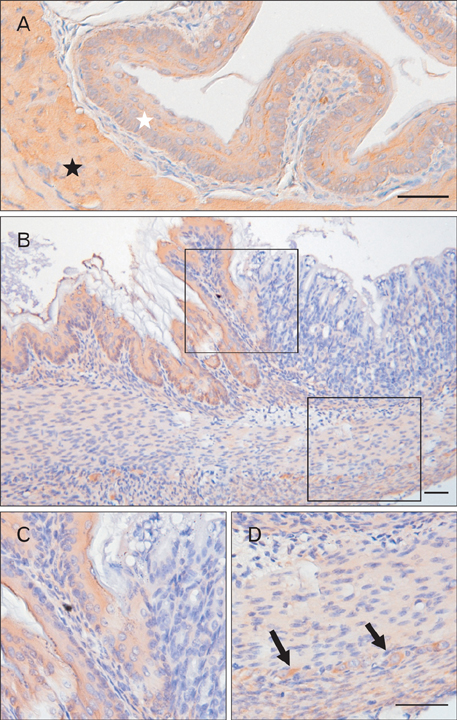
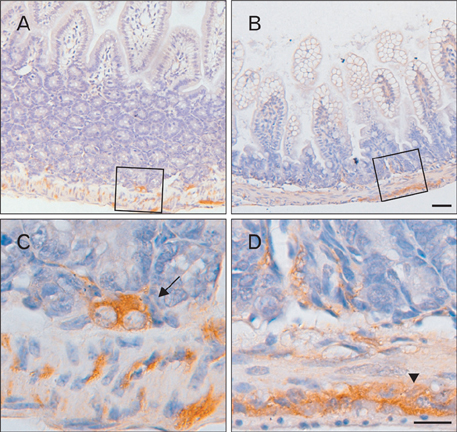
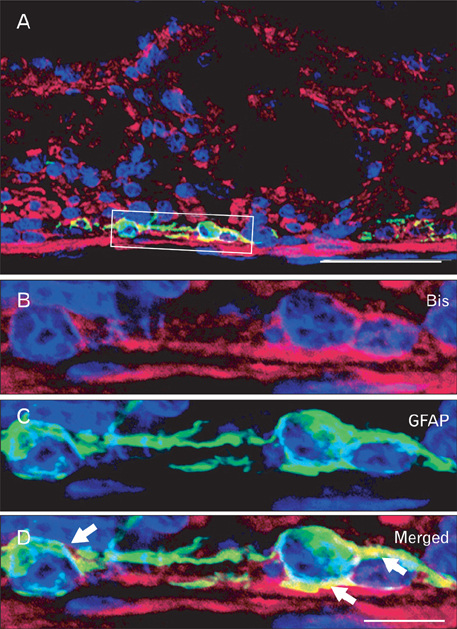
- The Bcl-2 interacting death suppressor (Bis) protein is known to be involved in a variety of pathophysiological conditions. We recently generated bis-deficient mice, which exhibited early lethality with typical nutritional deprivation status. To further investigate the molecular basis for the malnutrition phenotype of bis deficient mice, we explored Bis expression in the digestive system of normal mice. Western blot analysis and quantitative real time reverse transcription polymerase chain reaction analysis indicated that Bis expression is highest in the esophagus, followed by the stomach, colon, jejunum and ileum. Immunohistochemical data indicated that Bis expression is restricted to the stratified squamous epitheliums in the esophagus and forestomach, and was not notable in the columnar epitheliums in the stomach, small intestine and colon. In addition, strong Bis immunoreactivity was detected in the striated muscles surrounding the esophagus and smooth muscles at a lesser intensity throughout the gastrointestinal (GI) tract. Ganglionated plexuses, located in submucous layers, as well as intermuscular layers, were specifically immunoreactive for Bis. Immunofluorescence studies revealed that Bis is co-localized in glial fibrillary acidic protein-expressing enteric glial cells. Immunostaining with neuron specific esterase antibodies indicate that Bis is also present in the cell bodies of ganglions in the enteric nervous system (ENS). Our findings indicate that Bis plays a role in regulating GI functions, such as motility and absorption, through modulating signal transmission between the ENS and smooth muscles or the intestinal epitheliums.
MeSH Terms
-
Absorption
Animals
Antibodies
Blotting, Western
Colon
Digestive System
Enteric Nervous System
Epithelium
Esophagus
Fluorescent Antibody Technique
Ganglion Cysts
Ileum
Immunohistochemistry
Intestinal Mucosa
Intestine, Small
Jejunum
Malnutrition
Mice
Muscle, Smooth
Muscle, Striated
Neuroglia
Neurons
Phenotype
Polymerase Chain Reaction
Reverse Transcription
Stomach
Antibodies
Figure
Reference
-
1. Lee JH, Takahashi T, Yasuhara N, Inazawa J, Kamada S, Tsujimoto Y. Bis, a Bcl-2-binding protein that synergizes with Bcl-2 in preventing cell death. Oncogene. 1999. 18:6183–6190.2. Takayama S, Xie Z, Reed JC. An evolutionarily conserved family of Hsp70/Hsc70 molecular chaperone regulators. J Biol Chem. 1999. 274:781–786.3. Liao Q, Ozawa F, Friess H, Zimmermann A, Takayama S, Reed JC, Kleeff J, Büchler MW. The anti-apoptotic protein BAG-3 is overexpressed in pancreatic cancer and induced by heat stress in pancreatic cancer cell lines. FEBS Lett. 2001. 503:151–157.4. Romano MF, Festa M, Pagliuca G, Lerose R, Bisogni R, Chiurazzi F, Storti G, Volpe S, Venuta S, Turco MC, Leone A. BAG3 protein controls B-chronic lymphocytic leukaemia cell apoptosis. Cell Death Differ. 2003. 10:383–385.5. Chiappetta G, Ammirante M, Basile A, Rosati A, Festa M, Monaco M, Vuttariello E, Pasquinelli R, Arra C, Zerilli M, Todaro M, Stassi G, Pezzullo L, Gentilella A, Tosco A, Pascale M, Marzullo L, Belisario MA, Turco MC, Leone A. The antiapoptotic protein BAG3 is expressed in thyroid carcinomas and modulates apoptosis mediated by tumor necrosis factor-related apoptosis-inducing ligand. J Clin Endocrinol Metab. 2007. 92:1159–1163.6. Staibano S, Mascolo M, Di Benedetto M, Vecchione ML, Ilardi G, Di Lorenzo G, Autorino R, Salerno V, Morena A, Rocco A, Turco MC, Morelli E. BAG3 protein delocalisation in prostate carcinoma. Tumour Biol. 2010. 31:461–469.7. Ammirante M, De Laurenzi V, Graziano V, Turco MC, Rosati A. BAG3 is required for IKKalpha nuclear translocation and emergence of castration resistant prostate cancer. Cell Death Dis. 2011. 2:e139.8. Festa M, Del Valle L, Khalili K, Franco R, Scognamiglio G, Graziano V, De Laurenzi V, Turco MC, Rosati A. BAG3 protein is overexpressed in human glioblastoma and is a potential target for therapy. Am J Pathol. 2011. 178:2504–2512.9. Franco R, Scognamiglio G, Salerno V, Sebastiani A, Cennamo G, Ascierto PA, Botti G, Turco MC, Rosati A. Expression of the anti-apoptotic protein BAG3 in human melanomas. J Invest Dermatol. 2012. 132:252–254.10. Liu P, Xu B, Li J, Lu H. BAG3 gene silencing sensitizes leukemic cells to Bortezomib-induced apoptosis. FEBS Lett. 2009. 583:401–406.11. Wang HQ, Liu BQ, Gao YY, Meng X, Guan Y, Zhang HY, Du ZX. Inhibition of the JNK signalling pathway enhances proteasome inhibitor-induced apoptosis of kidney cancer cells by suppression of BAG3 expression. Br J Pharmacol. 2009. 158:1405–1412.12. Lee MY, Kim SY, Shin SL, Choi YS, Lee JH, Tsujimoto Y, Lee JH. Reactive astrocytes express bis, a bcl-2-binding protein, after transient forebrain ischemia. Exp Neurol. 2002. 175:338–346.13. Lee MY, Kim SY, Choi JS, Choi YS, Jeon MH, Lee JH, Kim IK, Lee JH. Induction of Bis, a Bcl-2-binding protein, in reactive astrocytes of the rat hippocampus following kainic acid-induced seizure. Exp Mol Med. 2002. 34:167–171.14. Pagliuca MG, Lerose R, Cigliano S, Leone A. Regulation by heavy metals and temperature of the human BAG-3 gene, a modulator of Hsp70 activity. FEBS Lett. 2003. 541:11–15.15. Rosati A, Leone A, Del Valle L, Amini S, Khalili K, Turco MC. Evidence for BAG3 modulation of HIV-1 gene transcription. J Cell Physiol. 2007. 210:676–683.16. Jung SE, Kim YK, Youn DY, Lim MH, Ko JH, Ahn YS, Lee JH. Down-modulation of Bis sensitizes cell death in C6 glioma cells induced by oxygen-glucose deprivation. Brain Res. 2010. 1349:1–10.17. Carra S, Seguin SJ, Lambert H, Landry J. HspB8 chaperone activity toward poly(Q)-containing proteins depends on its association with Bag3, a stimulator of macroautophagy. J Biol Chem. 2008. 283:1437–1444.18. Gamerdinger M, Hajieva P, Kaya AM, Wolfrum U, Hartl FU, Behl C. Protein quality control during aging involves recruitment of the macroautophagy pathway by BAG3. EMBO J. 2009. 28:889–901.19. Gamerdinger M, Kaya AM, Wolfrum U, Clement AM, Behl C. BAG3 mediates chaperone-based aggresome-targeting and selective autophagy of misfolded proteins. EMBO Rep. 2011. 12:149–156.20. Zhang X, Qian SB. Chaperone-mediated hierarchical control in targeting misfolded proteins to aggresomes. Mol Biol Cell. 2011. 22:3277–3288.21. Homma S, Iwasaki M, Shelton GD, Engvall E, Reed JC, Takayama S. BAG3 deficiency results in fulminant myopathy and early lethality. Am J Pathol. 2006. 169:761–773.22. Youn DY, Lee DH, Lim MH, Yoon JS, Lim JH, Jung SE, Yeum CE, Park CW, Youn HJ, Lee JS, Lee SB, Ikawa M, Okabe M, Tsujimoto Y, Lee JH. Bis deficiency results in early lethality with metabolic deterioration and involution of spleen and thymus. Am J Physiol Endocrinol Metab. 2008. 295:E1349–E1357.23. Youn DY, Yoon JS, Kim YK, Yeum CE, Lee SB, Youn HJ, Tsujimoto Y, Lee JH. Deletion of the bis gene results in a marked increase in the production of corticosterone that is associated with thymic atrophy in mice. Am J Physiol Endocrinol Metab. 2011. 301:E223–E231.24. Park HJ, Choi JS, Chun MH, Chung JW, Jeon MH, Lee JH, Lee MY. Immunohistochemical localization of Bis protein in the rat central nervous system. Cell Tissue Res. 2003. 314:207–214.25. Choi JS, Lee JH, Kim HY, Chun MH, Chung JW, Lee MY. Developmental expression of Bis protein in the cerebral cortex and hippocampus of rats. Brain Res. 2006. 1092:69–78.26. Choi JS, Lee JH, Shin YJ, Lee JY, Yun H, Chun MH, Lee MY. Transient expression of Bis protein in midline radial glia in developing rat brainstem and spinal cord. Cell Tissue Res. 2009. 337:27–36.27. Yoon JS, Lee MY, Lee JS, Park CS, Youn HJ, Lee JH. Bis is involved in glial differentiation of p19 cells induced by retinoic Acid. Korean J Physiol Pharmacol. 2009. 13:251–256.28. Goyal RK, Hirano I. The enteric nervous system. N Engl J Med. 1996. 334:1106–1115.29. Jessen KR. Glial cells. Int J Biochem Cell Biol. 2004. 36:1861–1867.30. Wood JD. Johnson LR, editor. Physiology of the enteric nervous system. Physiology of the Gastrointestinal Tract. 1994. Vol. 1:3rd ed. New York: Rven Press;423–482.31. Kunze WA, Furness JB. The enteric nervous system and regulation of intestinal motility. Annu Rev Physiol. 1999. 61:117–142.32. Hayashi H, Suzuki T, Yamamoto T, Suzuki Y. Cholinergic inhibition of electrogenic sodium absorption in the guinea pig distal colon. Am J Physiol Gastrointest Liver Physiol. 2003. 284:G617–G628.33. Cooke HJ, Reddix RA. Johnson LR, editor. Neural regulation of intestinal electrolyte transport. Physiology of the Gastrointestinal Tract. 1994. New York: Raven Press;2083–2132.34. Selcen D, Muntoni F, Burton BK, Pegoraro E, Sewry C, Bite AV, Engel AG. Mutation in BAG3 causes severe dominant childhood muscular dystrophy. Ann Neurol. 2009. 65:83–89.35. Lee HC, Cherk SW, Chan SK, Wong S, Tong TW, Ho WS, Chan AY, Lee KC, Mak CM. BAG3-related myofibrillar myopathy in a Chinese family. Clin Genet. 2012. 81:394–398.36. Yang Y, Allen E, Ding J, Wang W. Giant axonal neuropathy. Cell Mol Life Sci. 2007. 64:601–609.37. Fabrizi GM, Cavallaro T, Angiari C, Cabrini I, Taioli F, Malerba G, Bertolasi L, Rizzuto N. Charcot-Marie-Tooth disease type 2E, a disorder of the cytoskeleton. Brain. 2007. 130(Pt 2):394–403.38. Jaffer F, Murphy SM, Scoto M, Healy E, Rossor AM, Brandner S, Phadke R, Selcen D, Jungbluth H, Muntoni F, Reilly MM. BAG3 mutations: another cause of giant axonal neuropathy. J Peripher Nerv Syst. 2012. 17:210–216.39. Furness JB, Kunze WA, Clerc N. Nutrient tasting and signaling mechanisms in the gut. II. The intestine as a sensory organ: neural, endocrine, and immune responses. Am J Physiol. 1999. 277(5 Pt 1):G922–G928.40. Raybould HE, Glatzle J, Freeman SL, Whited K, Darcel N, Liou A, Bohan D. Detection of macronutrients in the intestinal wall. Auton Neurosci. 2006. 125:28–33.41. Baglole CJ, Davison JS, Meddings JB. Epithelial distribution of neural receptors in the guinea pig small intestine. Can J Physiol Pharmacol. 2005. 83:389–395.42. Berlioz F, Maoret JJ, Paris H, Laburthe M, Farinotti R, Rozé C. Alpha(2)-adrenergic receptors stimulate oligopeptide transport in a human intestinal cell line. J Pharmacol Exp Ther. 2000. 294:466–472.43. Cooke HJ. Neurobiology of the intestinal mucosa. Gastroenterology. 1986. 90:1057–1081.44. Kimm MH, Hardin JA, Gall DG. The role of nitric oxide in the regulation of macromolecular transport in rat jejunum. J Physiol. 1996. 490(Pt 1):243–248.45. Wong TP, Debnam ES, Leung PS. Involvement of an enterocyte renin-angiotensin system in the local control of SGLT1-dependent glucose uptake across the rat small intestinal brush border membrane. J Physiol. 2007. 584(Pt 2):613–623.46. Barry MK, Aloisi JD, Yeo CJ. Luminal adrenergic agonists modulate ileal transport by a local mechanism. J Surg Res. 1993. 54:603–609.47. Ishikawa Y, Eguchi T, Ishida H. Mechanism of beta-adrenergic agonist-induced transmural transport of glucose in rat small intestine. Regulation of phosphorylation of SGLT1 controls the function. Biochim Biophys Acta. 1997. 1357:306–318.48. Kreydiyyeh SI. Alpha and beta adrenoceptors mediate the inhibitory effect of epinephrine on the mucosal uptake of phenylalanine in the rat jejunum. Can J Physiol Pharmacol. 1997. 75:1312–1315.49. Bassotti G, Villanacci V, Fisogni S, Rossi E, Baronio P, Clerici C, Maurer CA, Cathomas G, Antonelli E. Enteric glial cells and their role in gastrointestinal motor abnormalities: introducing the neuro-gliopathies. World J Gastroenterol. 2007. 13:4035–4041.50. Rühl A, Nasser Y, Sharkey KA. Enteric glia. Neurogastroenterol Motil. 2004. 16:Suppl 1. 44–49.51. Bush TG, Savidge TC, Freeman TC, Cox HJ, Campbell EA, Mucke L, Johnson MH, Sofroniew MV. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell. 1998. 93:189–201.52. Sánchez MP, Silos-Santiago I, Frisén J, He B, Lira SA, Barbacid M. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature. 1996. 382:70–73.53. Aubé AC, Cabarrocas J, Bauer J, Philippe D, Aubert P, Doulay F, Liblau R, Galmiche JP, Neunlist M. Changes in enteric neurone phenotype and intestinal functions in a transgenic mouse model of enteric glia disruption. Gut. 2006. 55:630–637.54. Nasser Y, Fernandez E, Keenan CM, Ho W, Oland LD, Tibbles LA, Schemann M, MacNaughton WK, Rühl A, Sharkey KA. Role of enteric glia in intestinal physiology: effects of the gliotoxin fluorocitrate on motor and secretory function. Am J Physiol Gastrointest Liver Physiol. 2006. 291:G912–G927.55. Won KJ, Suzuki T, Hori M, Ozaki H. Motility disorder in experimentally obstructed intestine: relationship between muscularis inflammation and disruption of the ICC network. Neurogastroenterol Motil. 2006. 18:53–61.56. Bassotti G, Villanacci V, Maurer CA, Fisogni S, Di Fabio F, Cadei M, Morelli A, Panagiotis T, Cathomas G, Salerni B. The role of glial cells and apoptosis of enteric neurones in the neuropathology of intractable slow transit constipation. Gut. 2006. 55:41–46.57. Bassotti G, Villanacci V, Cathomas G, Maurer CA, Fisogni S, Cadei M, Baron L, Morelli A, Valloncini E, Salerni B. Enteric neuropathology of the terminal ileum in patients with intractable slow-transit constipation. Hum Pathol. 2006. 37:1252–1258.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Study on Eating Behavior, Depression, Anger, Anger Expression and BAS/BIS in Adolescent Women
- Bis is Induced by Oxidative Stress via Activation of HSF1
- Bis induces growth inhibition and differentiation of HL-60 cells via up-regulation of p27
- Myosin heavy chain is stabilized by BCL-2 interacting cell death suppressor (BIS) in skeletal muscle
- The Relation between Tourniquet Hypertension and Bispectral Index in Patients Undergoing a Total Knee Arthroplasty