J Korean Neurosurg Soc.
2014 Nov;56(5):375-382. 10.3340/jkns.2014.56.5.375.
Intra-Spinal Bone Marrow Mononuclear Cells Transplantation Inhibits the Expression of Nuclear Factor-kappaB in Acute Transection Spinal Cord Injury in Rats
- Affiliations
-
- 1Department of Orthopaedics, First Affiliated Hospital of Jiamusi University, Heilongjiang, China. jianhua01@163.com
- KMID: 2018094
- DOI: http://doi.org/10.3340/jkns.2014.56.5.375
Abstract
OBJECTIVE
To assess the effect of bone marrow mononuclear cells (BMMNCs) transplantation in the expression of nuclear factor-kappaB (NF-kappaB) in spinal cord injury (SCI) in rats.
METHODS
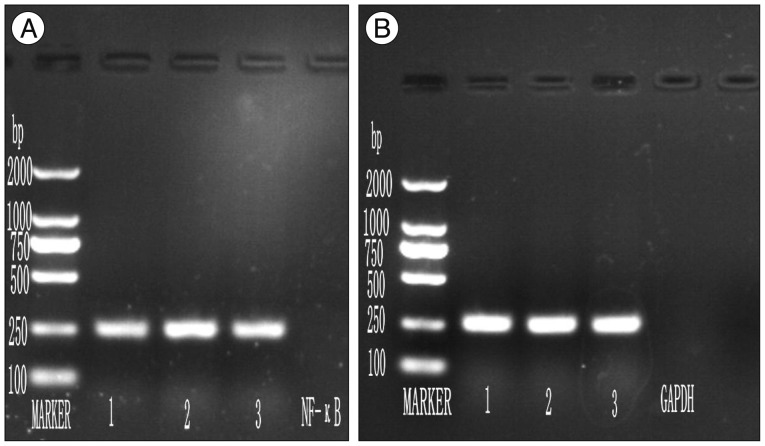
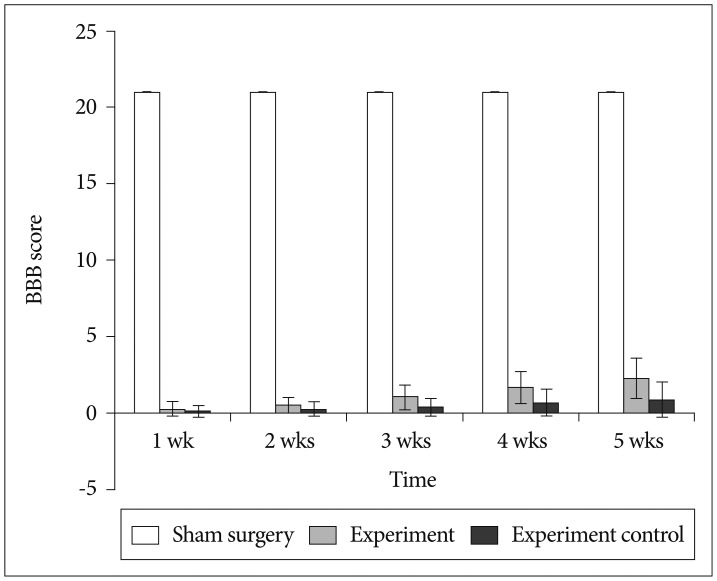
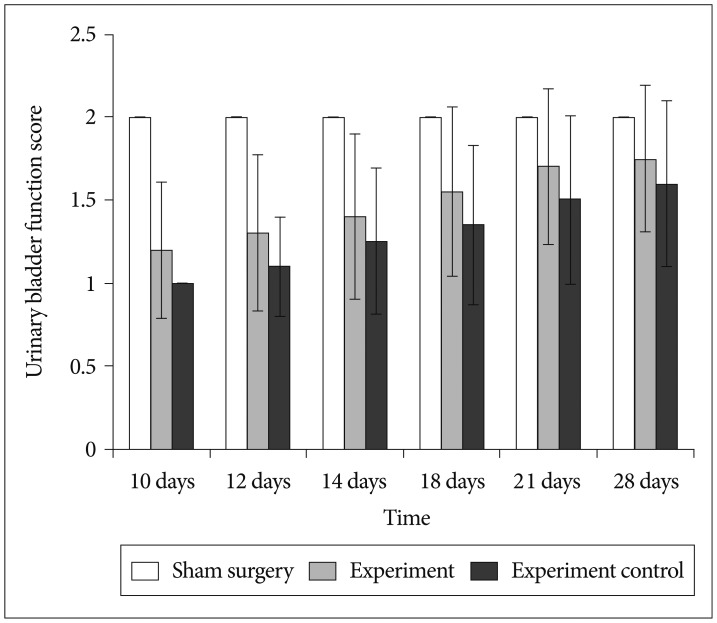
BMMNCs were isolated from tibia and femur by a density gradient centrifugation. After establishment of acute transection SCI, rats were divided into experiment (BMMNCs), experiment control (0.1 M PBS infused) and sham surgery groups (laminectomy without any SCI). Locomotor function was assessed weekly for 5 weeks post-injury using BBB locomotor score and urinary bladder function daily for 4 weeks post-injury. Activity of NF-kappaB in spinal cord was assessed by immunohistochemistry and reverse transcriptase polymerase chain reaction.
RESULTS
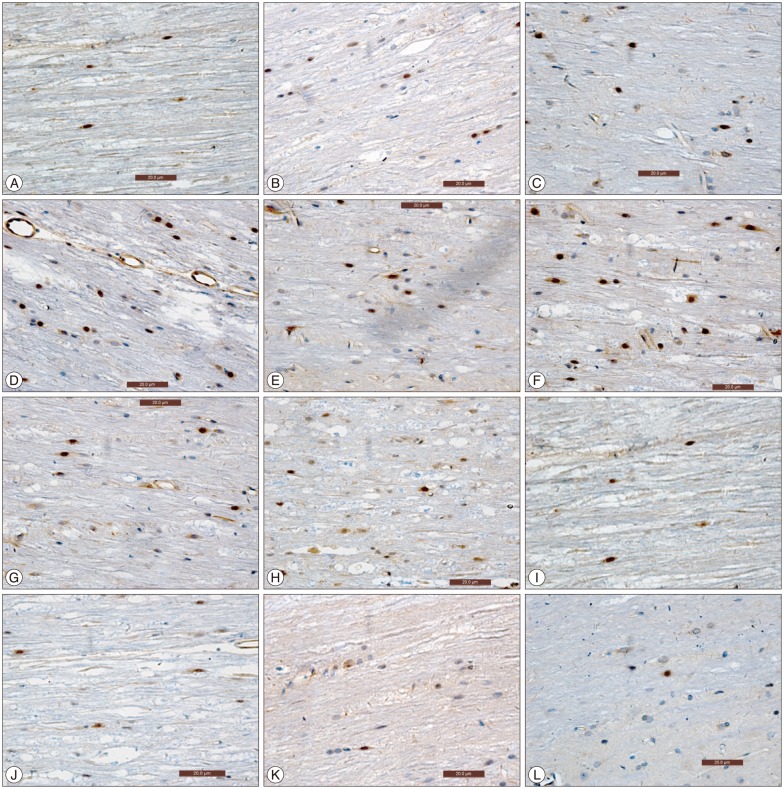
At each time point post-injury, sham surgery group had significantly higher Basso, Beattie, Bresnahan locomotor and urinary bladder function scores than experiment and experiment control group (p<0.05). At subsequent time interval there were gradual improvement in both experiment and experiment control group, but experiment group had higher score in comparison to experiment control group (p<0.05). Comparisons were also made for expression of activated NF-kappaB positive cells and level of NF-kappaB messenger RNA in spinal cord at various time points between the groups. Activated NF-kappaB immunoreactivity and level of NF-kappaB mRNA expression were significantly higher in control group in comparison to experiment and sham surgery group (p<0.05).
CONCLUSION
BMMNCs transplantation attenuates the expression of NF-kappaB in injured spinal cord tissue and thus helps in recovery of neurological function in rat models with SCI.
Keyword
MeSH Terms
Figure
Reference
-
1. Baeuerle PA. The inducible transcription activator NF-kappa B : regulation by distinct protein subunits. Biochim Biophys Acta. 1991; 1072:63–80. PMID: 2018779.
Article2. Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995; 12:1–21. PMID: 7783230.
Article3. Beattie MS. Inflammation and apoptosis : linked therapeutic targets in spinal cord injury. Trends Mol Med. 2004; 10:580–583. PMID: 15567326.
Article4. Bethea JR, Castro M, Keane RW, Lee TT, Dietrich WD, Yezierski RP. Traumatic spinal cord injury induces nuclear factor-kappaB activation. J Neurosci. 1998; 18:3251–3260. PMID: 9547234.5. Brambilla R, Bracchi-Ricard V, Hu WH, Frydel B, Bramwell A, Karmally S, et al. Inhibition of astroglial nuclear factor kappaB reduces inflammation and improves functional recovery after spinal cord injury. J Exp Med. 2005; 202:145–156. PMID: 15998793.
Article6. Brambilla R, Hurtado A, Persaud T, Esham K, Pearse DD, Oudega M, et al. Transgenic inhibition of astroglial NF-kappa B leads to increased axonal sparing and sprouting following spinal cord injury. J Neurochem. 2009; 110:765–778. PMID: 19522780.
Article7. Cao HQ, Dong ED. An update on spinal cord injury research. Neurosci Bull. 2013; 29:94–102. PMID: 23124646.
Article8. Chen LF, Greene WC. Shaping the nuclear action of NF-kappaB. Nat Rev Mol Cell Biol. 2004; 5:392–401. PMID: 15122352.9. Esposito E, Paterniti I, Mazzon E, Genovese T, Galuppo M, Meli R, et al. MK801 attenuates secondary injury in a mouse experimental compression model of spinal cord trauma. BMC Neurosci. 2011; 12:31. PMID: 21492450.
Article10. Genovese T, Paterniti I, Mazzon E, Esposito E, Di Paola R, Galuppo M, et al. Efficacy of treatment with verbascoside, biotechnologically produced by Syringa vulgaris plant cell cultures in an experimental mice model of spinal cord trauma. Naunyn Schmiedebergs Arch Pharmacol. 2010; 382:331–345. PMID: 20799028.
Article11. Glazova M, Pak ES, Moretto J, Hollis S, Brewer KL, Murashov AK. Pre-differentiated embryonic stem cells promote neuronal regeneration by cross-coupling of BDNF and IL-6 signaling pathways in the host tissue. J Neurotrauma. 2009; 26:1029–1042. PMID: 19138107.
Article12. Hamada Y, Ikata T, Katoh S, Tsuchiya K, Niwa M, Tsutsumishita Y, et al. Roles of nitric oxide in compression injury of rat spinal cord. Free Radic Biol Med. 1996; 20:1–9. PMID: 8903674.
Article13. Han X, Lu M, Wang S, Lv D, Liu H. Targeting IKK/NF-κB pathway reduces infiltration of inflammatory cells and apoptosis after spinal cord injury in rats. Neurosci Lett. 2012; 511:28–32. PMID: 22289688.
Article14. Han X, Wang S, Zhang Z, Lü D, Liu H. BMS-345541 inhibited nuclear factor kappa B expression and improved locomotor function recovery in rats after acute spinal cord injury. Neural Regen Res. 2011; 6:1775–1779.15. Hausmann ON. Post-traumatic inflammation following spinal cord injury. Spinal Cord. 2003; 41:369–378. PMID: 12815368.
Article16. Jia Z, Zhu H, Li J, Wang X, Misra H, Li Y. Oxidative stress in spinal cord injury and antioxidant-based intervention. Spinal Cord. 2012; 50:264–274. PMID: 21987065.
Article17. Kerr BJ, Girolami EI, Ghasemlou N, Jeong SY, David S. The protective effects of 15-deoxy-delta-(12,14)-prostaglandin J2 in spinal cord injury. Glia. 2008; 56:436–448. PMID: 18205174.
Article18. Kim GM, Xu J, Xu J, Song SK, Yan P, Ku G, et al. Tumor necrosis factor receptor deletion reduces nuclear factor-kappaB activation, cellular inhibitor of apoptosis protein 2 expression, and functional recovery after traumatic spinal cord injury. J Neurosci. 2001; 21:6617–6625. PMID: 11517251.
Article19. La Rosa G, Cardali S, Genovese T, Conti A, Di Paola R, La Torre D, et al. Inhibition of the nuclear factor-kappaB activation with pyrrolidine dithiocarbamate attenuating inflammation and oxidative stress after experimental spinal cord trauma in rats. J Neurosurg Spine. 2004; 1:311–321. PMID: 15478370.
Article20. Lee KM, Jeon SM, Cho HJ. Tumor necrosis factor receptor 1 induces interleukin-6 upregulation through NF-kappaB in a rat neuropathic pain model. Eur J Pain. 2009; 13:794–806. PMID: 18938092.
Article21. Liebscher T, Schnell L, Schnell D, Scholl J, Schneider R, Gullo M, et al. Nogo-A antibody improves regeneration and locomotion of spinal cord-injured rats. Ann Neurol. 2005; 58:706–719. PMID: 16173073.
Article22. Mandalari G, Genovese T, Bisignano C, Mazzon E, Wickham MS, Di Paola R, et al. Neuroprotective effects of almond skins in experimental spinal cord injury. Clin Nutr. 2011; 30:221–233. PMID: 20864228.
Article23. Mao L, Wang H, Qiao L, Wang X. Disruption of Nrf2 enhances the upregulation of nuclear factor-kappaB activity, tumor necrosis factor-α, and matrix metalloproteinase-9 after spinal cord injury in mice. Mediators Inflamm. 2010; 2010:238321. PMID: 20862369.24. Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999; 18:6853–6866. PMID: 10602461.
Article25. Parr AM, Tator CH, Keating A. Bone marrow-derived mesenchymal stromal cells for the repair of central nervous system injury. Bone Marrow Transplant. 2007; 40:609–619. PMID: 17603514.
Article26. Paterniti I, Genovese T, Crisafulli C, Mazzon E, Di Paola R, Galuppo M, et al. Treatment with green tea extract attenuates secondary inflammatory response in an experimental model of spinal cord trauma. Naunyn Schmiedebergs Arch Pharmacol. 2009; 380:179–192. PMID: 19337722.
Article27. Pizzi M, Spano P. Distinct roles of diverse nuclear factor-kappaB complexes in neuropathological mechanisms. Eur J Pharmacol. 2006; 545:22–28. PMID: 16854410.
Article28. Pollock G, Pennypacker KR, Mémet S, Israël A, Saporta S. Activation of NF-kappaB in the mouse spinal cord following sciatic nerve transection. Exp Brain Res. 2005; 165:470–477. PMID: 15912368.
Article29. Rafati DS, Geissler K, Johnson K, Unabia G, Hulsebosch C, Nesic-Taylor O, et al. Nuclear factor-kappaB decoy amelioration of spinal cord injury-induced inflammation and behavior outcomes. J Neurosci Res. 2008; 86:566–580. PMID: 17918744.
Article30. Schwaninger M, Inta I, Herrmann O. NF-kappaB signalling in cerebral ischaemia . Biochem Soc Trans. 2006; 34(Pt 6):1291–1294. PMID: 17073804.31. Sekhon LH, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine (Phila Pa 1976). 2001; 26(24 Suppl):S2–S12. PMID: 11805601.
Article32. Sribnick EA, Wingrave JM, Matzelle DD, Wilford GG, Ray SK, Banik NL. Estrogen attenuated markers of inflammation and decreased lesion volume in acute spinal cord injury in rats. J Neurosci Res. 2005; 82:283–293. PMID: 16130149.
Article33. Tchivileva IE, Nackley AG, Qian L, Wentworth S, Conrad M, Diatchenko LB. Characterization of NF-kB-mediated inhibition of catechol-O-methyltransferase. Mol Pain. 2009; 5:13. PMID: 19291302.
Article34. The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. The People's Republic of China: The Ministry of Science and Technology of the People's Republic of China;2006.35. Verma IM. Nuclear factor (NF)-kappaB proteins : therapeutic targets. Ann Rheum Dis. 2004; 63(Suppl 2):ii57–ii61. PMID: 15479873.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Fate of Transplanted Bone Marrow Derived Mesenchymal Stem Cells Following Spinal Cord Injury in Rats by Transplantation Routes
- Treatment of Complete Spinal Cord Injury Patients Receiving Autologous Bone Marrow Cell Transplantation and Bone Marrow Stimulation with Granulocyte Macrophage-Colony Stimulating Factor: Report of Three Cases
- MR Imaging of Cord Transection without Skeletal Injury: A case Report
- Functional Recovery in Complete Spinal Cord Injury after Transplantation of Human Umbilical Cord Blood Cells in Rats
- The Effect of Spinal Cord Injury on Pituitary-Testicular Hormone Axis in Rats