Ann Clin Microbiol.
2015 Jun;18(2):37-43. 10.5145/ACM.2015.18.2.37.
Evaluation of Peptide Nucleic Acid Probe-Based Fluorescence In Situ Hybridization for the Detection of Mycobacterium tuberculosis Complex and Nontuberculous Mycobacteria in Clinical Respiratory Specimens
- Affiliations
-
- 1Department of Laboratory Medicine, Pusan National University School of Medicine, Yangsan, Korea. cchl@pusan.ac.kr
- KMID: 1971110
- DOI: http://doi.org/10.5145/ACM.2015.18.2.37
Abstract
- BACKGROUND
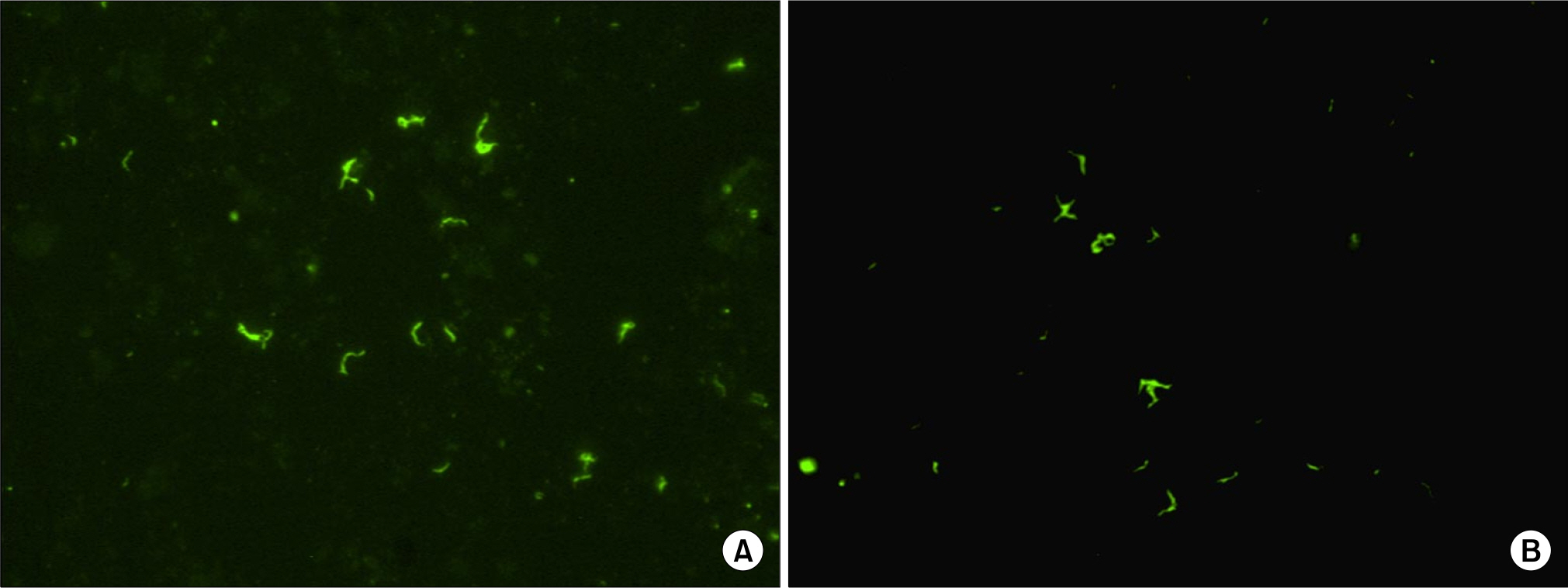
Tuberculosis is globally the most important cause of death from single pathogen. Rapid and accurate identification of mycobacteria is essential for the control of tuberculosis. We evaluated a fluorescence in situ hybridization (FISH) method using peptide nucleic acid (PNA) probes for the differentiation of Mycobacterium tuberculosis complex (MTB) and nontuberculous mycobacteria (NTM) in direct smears of sputum specimens.
METHODS
The cross-reactivity of MTB- and NTM-specific PNA probes was examined with reference strains of M. tuberculosis ATCC 13950, Mycobacterium kansasii ATCC 12479, Mycobacterium fortuitum ATCC 6841, several clinical isolates of mycobacteria (Mycobacterium abscessus, Mycobacterium avium, Mycobacterium intracellulare, Mycobacterium gordonae and Mycobacterium chelonae), and 11 frequently isolated respiratory bacterial species other than mycobacteria. A series of 128 sputa (89 MTB culture positive, 29 NTM culture positive, and 10 under treatment culture negative) with grades of trace to 4+ were used to evaluate the performance of the method.
RESULTS
The MTB- and NTM-specific PNA probes showed specific reactions with the reference strains of MTB and M. kansasii and clinical isolates of mycobacteria except M. fortuitum ATCC 6841, and no cross-reactivity with other tested bacteria. The PNA probe-based FISH assay for detection of MTB had a sensitivity and specificity of 100%, respectively. The sensitivity and specificity of the NTM-specific PNA probe was 100%. The smear grades of the PNA FISH test were same as with those of the fluorescence AFB stain in 2+ or higher grade.
CONCLUSION
Detection and differentiation based on PNA FISH is sensitive and accurate for detecting mycobacteria and for differentiating MTB from NTM in clinical sputum smears.
Keyword
MeSH Terms
Figure
Reference
-
1.WHO. WHO web sites on infectious diseases. Global Tuberculosis Report 2013.http://www.who.int/tb/publications/global_report/en/index.html/[Online. (last visited on 17 November 2014).2.Griffith DE., Aksamit T., Brown-Elliott BA., Catanzaro A., Daley C., Gordin F, et al. ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007. 175:367–416.
Article3.Richter E., Brown-Elliott BA., Wallace RJ. Mycobacterium: laboratory characteristics of slowly growing mycobacteria. Versalovic J, Carroll KC, Funke G, Jorgensen JH, Landry ML, Warnock DW, editors. Manual of Clinical Microbiology. 10th ed. Washington, DC: ASM Press;2011. p. 503–24.4.Shinnick TM., Iademarco MF., Ridderhof JC. National plan for reliable tuberculosis laboratory services using a systems approach. Recommendations from CDC and the Association of Public Health Laboratories Task Force on Tuberculosis Laboratory Services.5.Fitzgerald DW., Sterling TR., Haas DW. Mycobacterium tuberculosis. Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Disease. 7th ed. Philadelphia: Churchill Livingstone Elsevier;2010. p. 3129–63.6.Drobniewski FA., More PG., Harris GS. Differentiation of Mycobacterium tuberculosis complex and nontuberculous mycobacterial liquid cultures by using peptide nucleic acid-fluorescence in situ hybridization probes. J Clin Microbiol. 2000. 38:444–7.7.Padilla E., Manterola JM., Rasmussen OF., Lonca J., Domínguez J., Matas L, et al. Evaluation of a fluorescence hybridization assay using peptide nucleic acid probes for identification and differentiation of tuberculous and nontuberculous mycobacteria in liquid cultures. Eur J Clin Microbiol Infect Dis. 2000. 19:140–5.8.Stender H., Lund K., Petersen KH., Rasmussen OF., Hongmanee P., Miörner H, et al. Fluorescence in situ hybridization assay using peptide nucleic acid probes for differentiation between tuberculous and nontuberculous mycobacterium species in smears of mycobacterium cultures. J Clin Microbiol. 1999. 37:2760–5.
Article9.Stender H., Mollerup TA., Lund K., Petersen KH., Hongmanee P., Godtfredsen SE. Direct detection and identification of Mycobacterium tuberculosis in smear-positive sputum samples by fluorescence in situ hybridization (FISH) using peptide nucleic acid (PNA) probes. Int J Tuberc Lung Dis. 1999. 3:830–7.10.Choi YJ., Kim HJ., Shin HB., Nam HS., Lee SH., Park JS, et al. Evaluation of peptide nucleic acid probe-based real-time PCR for detection of Mycobacterium tuberculosis complex and nontuberculous mycobacteria in respiratory specimens. Ann Lab Med. 2012. 32:257–63.11.Rodriguez-Nuñez J., Avelar FJ., Marquez F., Rivas-Santiago B., Quiñones C., Guerrero-Barrera AL. Mycobacterium tuberculosis complex detected by modified fluorescent in situ hybridization in lymph nodes of clinical samples. J Infect Dev Ctries. 2012. 6:58–66.12.Lefmann M., Schweickert B., Buchholz P., Göbel UB., Ulrichs T., Seiler P, et al. Evaluation of peptide nucleic acid-fluorescence in situ hybridization for identification of clinically relevant mycobacteria in clinical specimens and tissue sections. J Clin Microbiol. 2006. 44:3760–7.
Article13.Choi YJ., Kim HS., Lee SH., Park JS., Nam HS., Kim HJ, et al. Evaluation of peptide nucleic acid array for the detection of hepatitis B virus mutations associated with antiviral resistance. Arch Virol. 2011. 156:1517–24.
Article14.Porcheddu A., Giacomelli G. Peptide nucleic acids (PNAs), a chemical overview. Curr Med Chem. 2005. 12:2561–99.
Article15.Egholm M., Nielsen PE., Buchardt O., Berg RH. Recognition of guanine and adenine in DNA by cytosine and thymine containing peptide nucleic acids (PNA). J Am Chem Soc. 1992. 114:9677–8.
Article16.Diagnostic Standards and Classification of Tuberculosis in Adults and Children. This official statement of the American Thoracic Society and the Centers for Disease Control and Prevention was adopted by the ATS Board of Directors, July 1999. This statement was endorsed by the Council of the Infectious Disease Society of America, September 1999. Am J Respir Crit Care Med. 2000. 161:1376–95.17.Hongmanee P., Stender H., Rasmussen OF. Evaluation of a fluorescence in situ hybridization assay for differentiation between tuberculous and nontuberculous Mycobacterium species in smears of Lowenstein-Jensen and Mycobacteria Growth Indicator Tube cultures using peptide nucleic acid probes. J Clin Microbiol. 2001. 39:1032–5.18.Perry-O'Keefe H., Rigby S., Oliveira K., S⊘rensen D., Stender H., Coull J, et al. Identification of indicator microorganisms using a standardized PNA FISH method. J Microbiol Methods. 2001. 47:281–92.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of Peptide Nucleic Acid Probe-based Real-time PCR for Detection of Mycobacterium tuberculosis Complex and Nontuberculous Mycobacteria in Respiratory Specimens
- Evaluation of Dual-Color Fluorescence In Situ Hybridization With Peptide Nucleic Acid Probes for the Detection of Mycobacterium tuberculosis and Non-Tuberculous Mycobacteria in Clinical Specimens
- Recent Advances in Tuberculosis and Nontuberculous Mycobacteria Lung Disease
- Evaluation of Two PCR-Hybridization Methods for the Detection of Mycobacterium tuberculosis
- Clinical utility of Amplified Mycobacterium tuberculosis Direct Test in the Diagnosis of Pulmonary Tuberculosis