J Korean Med Assoc.
2003 Mar;46(3):186-195. 10.5124/jkma.2003.46.3.186.
Nuclear Medicine in Oncology
- Affiliations
-
- 1Department of Nuclear Medicine, Korea Institute of Radiological and Medical Science, Korea. cwchoi@kcch.re.kr
- KMID: 1958054
- DOI: http://doi.org/10.5124/jkma.2003.46.3.186
Abstract
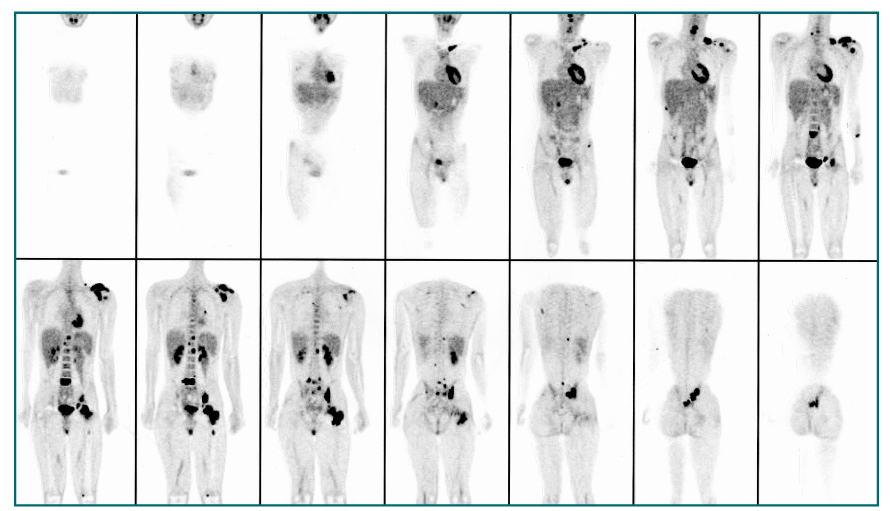
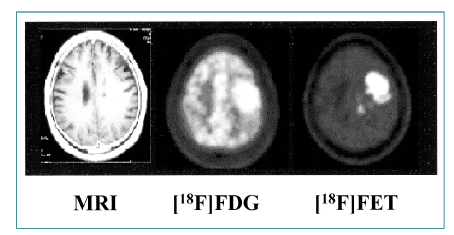
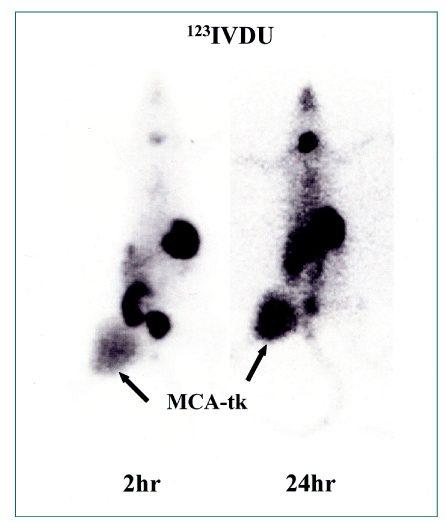
- Nuclear oncolgy is important in the diagnosis, staging, and long-term surveillance of a number of cancers. Over the past 10 years there has been an explosion of new radioisotopic tracers aimed at detecting, staging and eventually treating tumors. Clinicians and oncologists can now use specific radiolabeled metabolic tracers, monoclonal antibodies, and molecular probes based on the sequencing of the human genome. The current applications of positron emission tomography (PET) in oncology have included characterizing tumor lesions, differentiating recurrent disease from treatment effects, staging tumors, evaluating the extent of disease, and monitoring therapy. The future developments in medicine may use the unique capabilities of PET not only in diagnostic imaging but also in molecular medicine and genetics. Radioimmunoscintigraphy is a technique which uses radiolabeled antibodies to visualize tumors, taking advantage of antigens preferentially expressed by malignant tissue. However, the implementation of radiolabeled antibodies as "magic bullets" for detection and treatment of diseases such as cancer has required addressing several shortcomings of murine monoclonal antibodies. Genetic engineering provides a powerful approach for redesigning antibodies for use in oncologic applications in vivo. Recently, noninvasive molecular imaging has been developed. Most current molecular imaging strategies are "indirect" and involve the coupling of a "reporter gene" with a complementary "reporter probe". Imaging the level of probe accumulation provides indirect information related to the level of reporter gene expression. In this article, the author discuss the current status of PET, radioimmunoscintigraphy, gene imaging and receptor imaging with a brief review on nuclear oncology.
Keyword
MeSH Terms
Figure
Reference
-
5. Delbeke D, Martin WH. Positron emission tomography imaging in oncology. Radiol Clin North Am. 2001. 39:883–917.
Article6. Mankoff DA, Bellon JR. Positron-emission tomographic imaging of cancer: glucose metabolism and beyond. Semin Radiat Oncol. 2001. 11:16–27.
Article7. Anderson H, Price P. What does positron emission tomography offer oncology? Eur J Cancer. 2000. 36:2028–2035.
Article8. Bomanji JB, Costa DC, Ell PJ. Clinical role of positron emission tomography in oncology. Lancet Oncol. 2001. 2:157–164.
Article9. Laverman P, Boerman OC, Corstens FH, Oyen WJ. Fluorinated amino acids for tumour imaging with positron emission tomography. Eur J Nucl Med Mol Imaging. 2002. 29:681–690.
Article10. Fujiwara T, Matsuzawa T, Kubota K, Abe Y, Itoh M, Fukuda H, et al. Relationship between histologic type of primary lung cancer and carbon-11-L-methionine uptake with positron emission tomography. J Nucl Med. 1989. 30:33–37.11. Ishiwata K, Vaalburg W, Elsinga PH, Paans AM, Worldring MG. Comparison of L-[1-11C]methionine and L-methyl-[1-11C]methionine for measuring in vivo protein synthesis rates with PET. J Nucl Med. 1988. 29:1419–1427.12. Keen RE, Barrio JR, Huang SC, Hawkins RA, Phelps ME. In vivo cerebral protein synthesis rates with leucyl-transfer RNA used as a precursor pool: determination of biochemical parameters to structure tracer kinetic models for positron emission tomography. J Cereb Blood Flow Metab. 1989. 9:429–445.
Article13. Willemsen ATM, van Waarde A, Paans AMJ, Pruim J, Luurtsema G, Vaalburg W, et al. In vivo protein synthesis rate determination in primary or recurrent brain tumors using L-[1-11C]-tyrosine and PET. J Nucl Med. 1995. 36:411–419.14. Rasey JS, Grierson JR, Wiens LW, Kolb PD, Schwartz JL. Validation of FLT uptake as a measure of thymidine kinase -1 activity in A549 carcinoma cells. J Nucl Med. 2002. 43:1210–1217.15. Buck AK, Schirrmeister H, Hetzel M, Von Der Heide M, Halter G, Neumaier B, et al. 3-deoxy-3-[18F]fluorothymidine-positron emission tomography for noninvasive assessment of proliferation in pulmonary nodules. Cancer Res. 2002. 62(12):3331–3334.16. Mier W, Haberkorn U, Eisenhut M. [18F]FLT; portrait of a prolferation marker. Eur J Nucl Med Mol Imaging. 2002. 29:165–169.28. Tjuvajev JG, Avril N, Oku T, Sasajima T, Miyagawa T, Blasberg R, et al. Imaging herpes virus thymidine kinase gene transfer and expression by positron emission tomography. Cancer Res. 1998. 58(19):4333–4341.29. Tjuvajev JG, Finn R, Watanabe K, Joshi R, Oku T, Blasberg RG, et al. Noninvasive imaging of herpes virus thymidine kinase gene transfer and expression: a potential method for monitoring clinical gene therapy. Cancer Res. 1996. 56(18):4087–4095.30. Tjuvajev JG, Stockhammer G, Desai R, Uehara H, Watanabe K, Blasberg RG, et al. Imaging the expression of transfected genes in vivo. Cancer Res. 1995. 55(24):6126–6132.31. Gambhir SS, Barrio JR, Wu L, Iyer M, Namavari M, Herschman HR, et al. Imaging of adenoviral - directed herpes simplex virus type 1 thymidine kinase reporter gene expression in mice with radiolabeled ganciclovir. J Nucl Med. 1998. 39:2003–2011.32. Choi CW, Lang L, Lee JT, Webber KO, Yoo TM, Pastan I, et al. Biodistribution of 18F- and 125I-labeled anti-Tac disulfide-stabilized Fv fragments in nude mice with interleukin 2 alpha receptor - positive tumor xenografts. Cancer Res. 1995. 55(22):5323–5329.33. Yoo TM, Chang HK, Choi CW, Webber KO, Le N, Paik CH, et al. Technetium-99m labeling and biodistribution of anti-TAC disulfide-stabilized Fv fragment. J Nucl Med. 1997. 38:294–300.34. Wu AM, Yazaki PJ. Designer genes: recombinant antibody fragments for biological imaging. Q J Nucl Med. 2000. 44:268–283.35. Sergides IG, Austin RC, Winslet MC. Radioimmunodetection: technical problems and methods of improvement. Eur J Surg Oncol. 1999. 25:529–539.
Article36. Kalofonos HP, Karamouzis MV, Epenetos AA. Radioimmunoscintigraphy in patients with ovarian cancer. Acta Oncol. 2001. 40:549–557.
Article37. Berglund AS, Hulthen UL, Manhem P, Thorsson O, Wollmer P, Tornquist C. Metaiodobenzylguanidine (MIBG) scintigraphy and computed tomography (CT) in clinical practice. Primary and secondary evaluation for localization of phaeochromocytomas. J Intern Med. 2001. 249:247–251.
Article38. Sisson JC, Shulkin BL. Nuclear medicine imaging of pheochromocytoma and neuroblastoma. Q J Nucl Med. 1999. 43:217–223.39. Sisson JC. Radiopharmaceutical treatment of pheochromocytomas. Ann N Y Acad Sci. 2002. 970:54–60.
Article40. Bombardieri E, Maccauro M, De Deckere E, Savelli G, Chiti A. Nuclear medicine imaging of neuroendocrine tumours. Ann Oncol. 2001. 12:Suppl 2. S51–S61.
Article41. Signore A, Annovazzi A, Chianelli M, Corsetti F, Van de Wiele C, Watherhouse RN. Peptide radiopharmaceuticals for diagnosis and therapy. Eur J Nucl Med. 2001. 28:1555–1565.42. Behr TM, Gotthardt M, Barth A, Behe M. Imaging tumors with peptide-based radioligands. Q J Nucl Med. 2001. 45:189–200.43. Jonson SD, Welch MJ. PET imaging of breast cancer with fluorine - 18 radiolabeled estrogens and progestins. Q J Nucl Med. 1998. 42(1):8–17.