Korean Circ J.
2014 Jan;44(1):16-21. 10.4070/kcj.2014.44.1.16.
Effect of Hypoxic Paracrine Media on Calcium-Regulatory Proteins in Infarcted Rat Myocardium
- Affiliations
-
- 1Institute of Catholic Integrative Medicine, Incheon St. Mary's Hospital, The Catholic University of Korea College of Medicine, Incheon, Korea.
- 2Division of Cardiology, Severance Cardiovascular Research Center, Yonsei University College of Medicine, Seoul, Korea. mhlee@yuhs.ac
- KMID: 1859237
- DOI: http://doi.org/10.4070/kcj.2014.44.1.16
Abstract
- BACKGROUND AND OBJECTIVES
An increase in intracellular calcium concentration due to loss of Ca2+ homeostasis triggers arrhythmia or cardiac cell death in the heart. Paracrine factors released from stem cells have beneficial cardioprotective effects. However, the mechanism of modulation of Ca2+ homeostasis by paracrine factors in ischemic myocardium remains unclear.
MATERIALS AND METHODS
We isolated rat bone marrow-derived mesenchymal stem cells (MSCs), and prepared paracrine media (PM) from MSCs under hypoxic or normoxic conditions (hypoxic PM and normoxic PM). We induced rat myocardial infarction by left anterior descending ligation for 1 hour, and treated PM into the border region of infarcted myocardium (n=6/group) to identify the alteration in calcium-regulated proteins. We isolated and stained the heart tissue with specific calcium-related antibodies after 11 days.
RESULTS
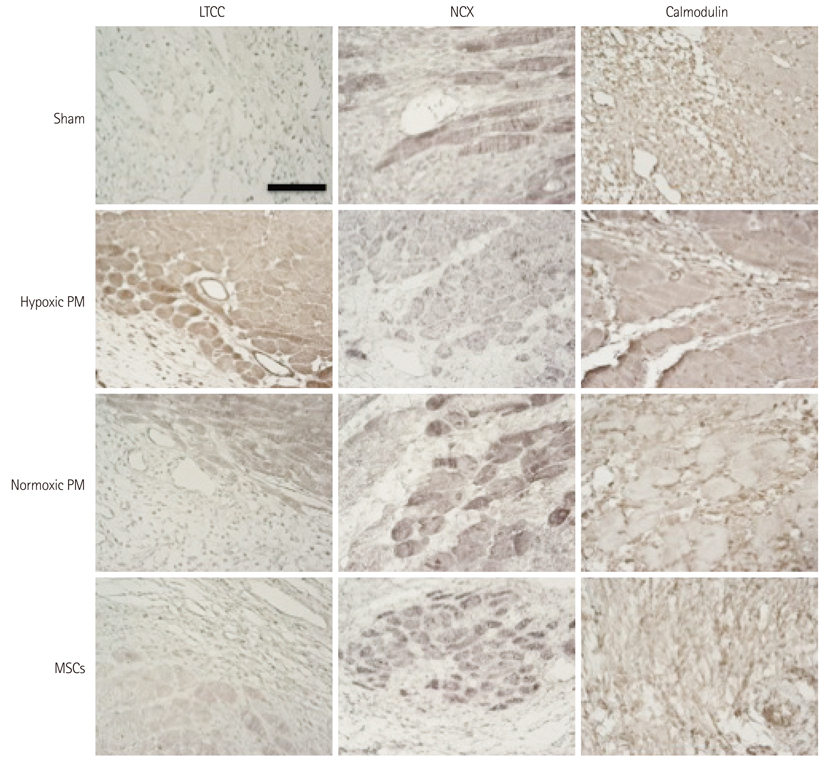
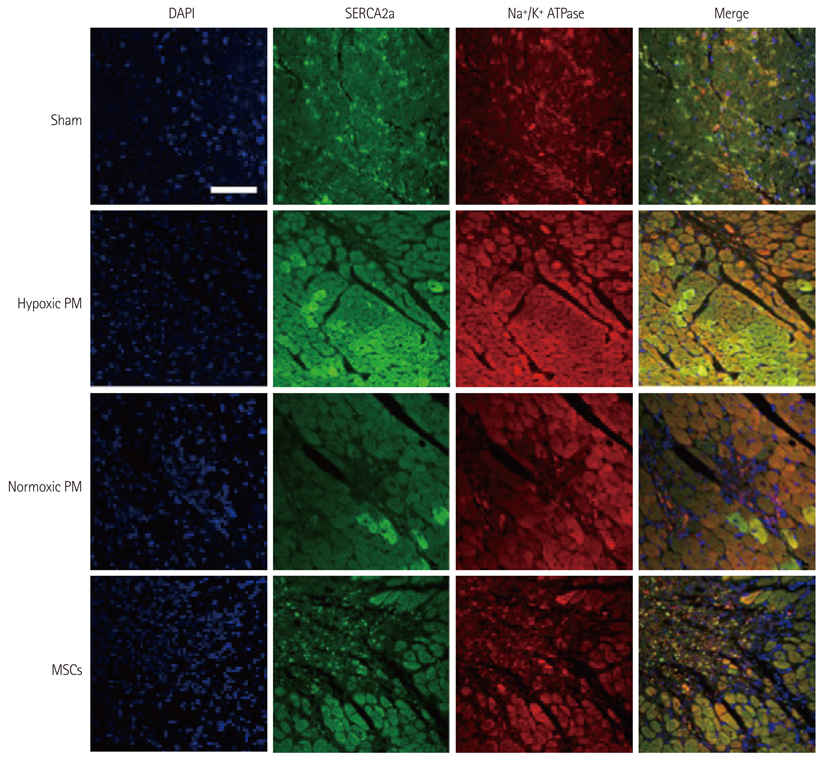
The hypoxic PM treatment increased Ca2+-related proteins such as L-type Ca2+ channel, sarcoplasmic reticulum Ca2+ ATPase, Na+/K+ ATPase, and calmodulin, whereas the normoxic PM treatment increased those proteins only slightly. The sodium-calcium exchanger was significantly reduced by hypoxic PM treatment, compared to moderate suppression by the normoxic PM treatment.
CONCLUSION
Our results suggest that hypoxic PM was significantly associated with the positive regulation of Ca2+ homeostasis in infarcted myocardium.
Keyword
MeSH Terms
-
Adenosine Triphosphatases
Animals
Antibodies
Arrhythmias, Cardiac
Calcium
Calcium-Transporting ATPases
Calmodulin
Cell Death
Heart
Homeostasis
Ligation
Mesenchymal Stromal Cells
Myocardial Infarction
Myocardium*
Paracrine Communication
Rats*
Sarcoplasmic Reticulum
Sodium-Calcium Exchanger
Stem Cells
Adenosine Triphosphatases
Antibodies
Calcium
Calcium-Transporting ATPases
Calmodulin
Sodium-Calcium Exchanger
Figure
Reference
-
1. Webster KA, Graham RM, Thompson JW, et al. Redox stress and the contributions of BH3-only proteins to infarction. Antioxid Redox Signal. 2006; 8:1667–1676.2. Orrenius S, McConkey DJ, Bellomo G, Nicotera P. Role of Ca2+ in toxic cell killing. Trends Pharmacol Sci. 1989; 10:281–285.3. Mathur A, Martin JF. Stem cells and repair of the heart. Lancet. 2004; 364:183–192.4. Couzin J, Vogel G. Cell therapy. Renovating the heart. Science. 2004; 304:192–194.5. Orlic D, Hill JM, Arai AE. Stem cells for myocardial regeneration. Circ Res. 2002; 91:1092–1102.6. Min JY, Sullivan MF, Yang Y, et al. Significant improvement of heart function by cotransplantation of human mesenchymal stem cells and fetal cardiomyocytes in postinfarcted pigs. Ann Thorac Surg. 2002; 74:1568–1575.7. Mangi AA, Noiseux N, Kong D, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003; 9:1195–1201.8. Song H, Chang W, Lim S, et al. Tissue transglutaminase is essential for integrin-mediated survival of bone marrow-derived mesenchymal stem cells. Stem Cells. 2007; 25:1431–1438.9. Chang MG, Tung L, Sekar RB, et al. Proarrhythmic potential of mesenchymal stem cell transplantation revealed in an in vitro coculture model. Circulation. 2006; 113:1832–1841.10. Nagaya N, Fujii T, Iwase T, et al. Intravenous administration of mesenchymal stem cells improves cardiac function in rats with acute myocardial infarction through angiogenesis and myogenesis. Am J Physiol Heart Circ Physiol. 2004; 287:H2670–H2676.11. Miyahara Y, Nagaya N, Kataoka M, et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. 2006; 12:459–465.12. Nagaya N, Kangawa K, Itoh T, et al. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation. 2005; 112:1128–1135.13. Hwang HJ, Chang W, Song BW, et al. Antiarrhythmic potential of mesenchymal stem cell is modulated by hypoxic environment. J Am Coll Cardiol. 2012; 60:1698–1706.14. Decker KF, Rudy Y. Ionic mechanisms of electrophysiological heterogeneity and conduction block in the infarct border zone. Am J Physiol Heart Circ Physiol. 2010; 299:H1588–H1597.15. Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008; 70:23–49.16. Sipido KR, Bito V, Antoons G, Volders PG, Vos MA. Na/Ca exchange and cardiac ventricular arrhythmias. Ann N Y Acad Sci. 2007; 1099:339–348.17. Venetucci LA, Trafford AW, O'Neill SC, Eisner DA. Na/Ca exchange: regulator of intracellular calcium and source of arrhythmias in the heart. Ann N Y Acad Sci. 2007; 1099:315–325.18. Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002; 105:93–98.19. Beeres SL, Atsma DE, van der Laarse A, et al. Human adult bone marrow mesenchymal stem cells repair experimental conduction block in rat cardiomyocyte cultures. J Am Coll Cardiol. 2005; 46:1943–1952.20. Berry MF, Engler AJ, Woo YJ, et al. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am J Physiol Heart Circ Physiol. 2006; 290:H2196–H2203.21. Gnecchi M, He H, Noiseux N, et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006; 20:661–669.22. Ostadal P, Elmoselhi AB, Zdobnicka I, Lukas A, Chapman D, Dhalla NS. Ischemia-reperfusion alters gene expression of Na+-K+ ATPase isoforms in rat heart. Biochem Biophys Res Commun. 2003; 306:457–462.23. Ehrlich BE, Kaftan E, Bezprozvannaya S, Bezprozvanny I. The pharmacology of intracellular Ca(2+)-release channels. Trends Pharmacol Sci. 1994; 15:145–149.24. Leszek P, Szperl M, Klisiewicz A, et al. Alteration of myocardial sarcoplasmic reticulum Ca2+-ATPase and Na+-Ca2+ exchanger expression in human left ventricular volume overload. Eur J Heart Fail. 2007; 9:579–586.25. Jeck CD, Zimmermann R, Schaper J, Schaper W. Decreased expression of calmodulin mRNA in human end-stage heart failure. J Mol Cell Cardiol. 1994; 26:99–107.26. Talukder MA, Kalyanasundaram A, Zuo L, et al. Is reduced SERCA2a expression detrimental or beneficial to postischemic cardiac function and injury? Evidence from heterozygous SERCA2a knockout mice. Am J Physiol Heart Circ Physiol. 2008; 294:H1426–H1434.27. Solomon SD, Zelenkofske S, McMurray JJ, et al. Sudden death in patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. N Engl J Med. 2005; 352:2581–2588.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Change of Expression of Troponin T and I Isoforms in the Failing Myocardium of Rat after Myocardial Infarction
- Effect of High Doses of Propofol on Myocardium in the Isolated Rat Heart
- Effect of composite of bone morphogenetic protein and plaster of paris on healing of bone defect in the rat tibia
- Liver Regenerating Potential of the Secretome Obtained from Adipose-derived Stem Cells Cultured under the Hypoxic Environment
- Effect of L-carnitine on ischemic myocardium of Langendorff's isolated rat heart