Anat Cell Biol.
2015 Jun;48(2):104-113. 10.5115/acb.2015.48.2.104.
The effects of repetitive transcranial magnetic stimulation on proliferation and differentiation of neural stem cells
- Affiliations
-
- 1Department of Physical Therapy, School of Rehabilitation Sciences, Shiraz University of Medical Sciences, Shiraz, Iran.
- 2Student Research Committee, Shiraz University of Medical Sciences, Shiraz, Iran.
- 3Neural Stem Cell and Regenerative Neuroscience Laboratory, Department of Anatomical Sciences, Shiraz School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran. azarihasan@sums.ac.ir
- 4Department of Physical Therapy, College of Public Health and Health Professions, University of Florida, Gainesville, FL, USA.
- 5Neural Stem Cell and Regenerative Neuroscience Laboratory, Shiraz Stem Cell Institute, Shiraz University of Medical Sciences, Shiraz, Iran.
- KMID: 1845278
- DOI: http://doi.org/10.5115/acb.2015.48.2.104
Abstract
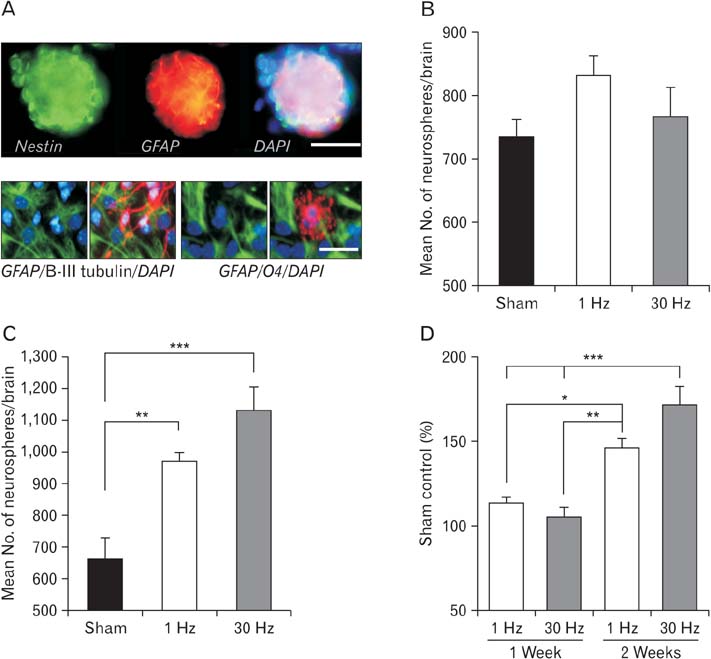
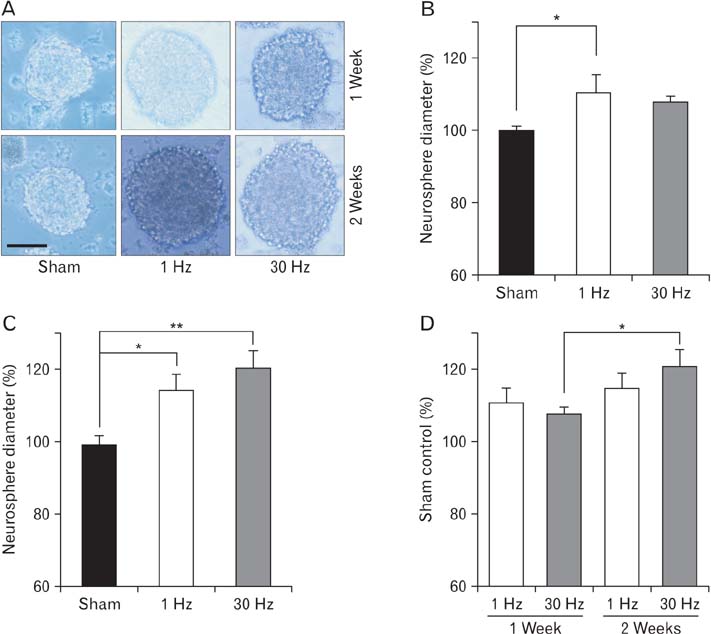
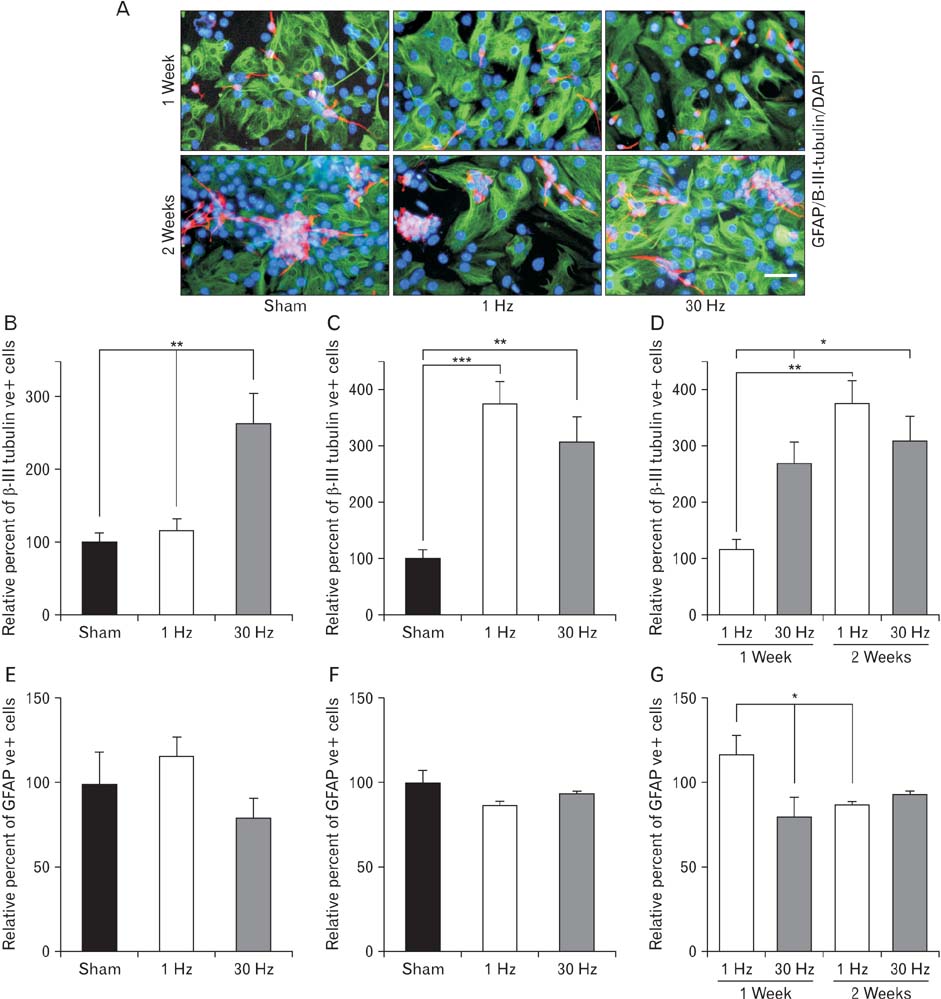
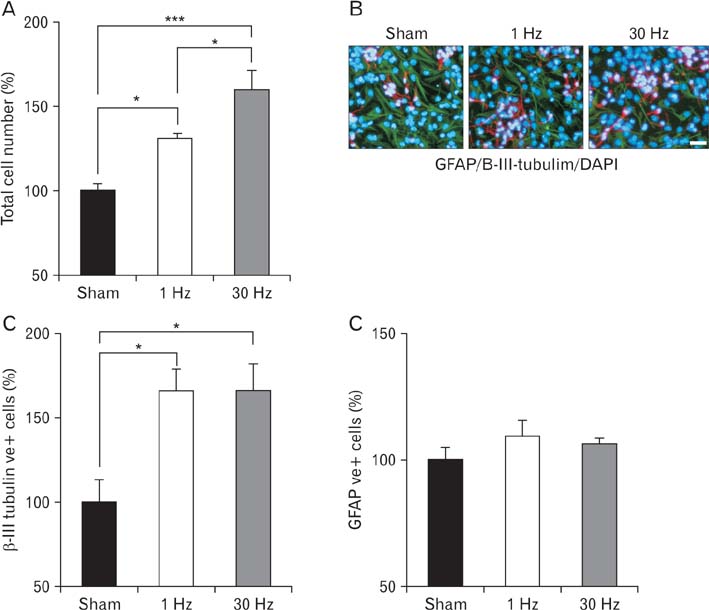
- Repetitive transcranial magnetic stimulation (rTMS) is a new method for treating many neurological conditions; however, the exact therapeutic mechanisms behind rTMS-induced plasticity are still unknown. Neural stem and progenitor cells (NS/PCs) are active players in brain regeneration and plasticity but their behavior in the context of rTMS therapy needs further elucidation. We aimed to evaluate the effects of rTMS on proliferation and differentiation of NS/PCs in the subventricular zone (SVZ) of adult mouse brain. Adult male mice (n=30) were divided into rTMS (1-Hz and 30-Hz) and sham groups and treated for 7 or 14 consecutive days. Harvested NS/PCs from the SVZ were cultured in the neurosphere assay for 8 days and the number and size of the resulting neurospheres as well as their in vitro differentiation capacity were evaluated. After one week of rTMS treatment at 1-Hz and 30-Hz compared with sham stimulation, the mean neurosphere forming frequency per brain was not different while this measure significantly increased after two weeks (P<0.05). The mean neurosphere diameter in 1-Hz treatment paradigm was significantly larger compared with sham stimulation at both 1 and 2 weeks. In contrast, 30-Hz treatment paradigm resulted in significantly larger neurospheres only after 2 weeks. Importantly, rTMS treatment at both frequencies increased neuronal differentiation of the harvested NS/PCs. Furthermore, one week in vitro rTMS treatment of NS/PCs with both 1-Hz and 30-Hz increased NS/PCs proliferation and neuronal differentiation. It is concluded that both 1-Hz and 30-Hz rTMS treatment increase NS/PCs proliferation and neuronal differentiation.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Safflower seed oil, a rich source of linoleic acid, stimulates hypothalamic neurogenesis in vivo
Mehrzad Jafari Barmak, Ebrahim Nouri, Maryam Hashemi Shahraki, Ghasem Ghalamfarsa, Kazem Zibara, Hamdallah Delaviz, Amir Ghanbari
Anat Cell Biol. 2023;56(2):219-227. doi: 10.5115/acb.22.220.
Reference
-
1. Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009; 120:2008–2039.2. Groppa S, Oliviero A, Eisen A, Quartarone A, Cohen LG, Mall V, Kaelin-Lang A, Mima T, Rossi S, Thickbroom GW, Rossini PM, Ziemann U, Valls-Solé J, Siebner HR. A practical guide to diagnostic transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol. 2012; 123:858–882.3. Arias-Carrion O. Basic mechanisms of rTMS: Implications in Parkinson's disease. Int Arch Med. 2008; 1:2.4. Le Q, Qu Y, Tao Y, Zhu S. Effects of repetitive transcranial magnetic stimulation on hand function recovery and excitability of the motor cortex after stroke: a meta-analysis. Am J Phys Med Rehabil. 2014; 93:422–430.5. Khedr EM, Ahmed MA, Fathy N, Rothwell JC. Therapeutic trial of repetitive transcranial magnetic stimulation after acute ischemic stroke. Neurology. 2005; 65:466–468.6. Lefaucheur JP. Stroke recovery can be enhanced by using repetitive transcranial magnetic stimulation (rTMS). Neurophysiol Clin. 2006; 36:105–115.7. Volz LJ, Benali A, Mix A, Neubacher U, Funke K. Dose-dependence of changes in cortical protein expression induced with repeated transcranial magnetic theta-burst stimulation in the rat. Brain Stimul. 2013; 6:598–606.8. Sczesny-Kaiser M, Bauknecht A, Höffken O, Tegenthoff M, Dinse HR, Jancke D, Funke K, Schwenkreis P. Synergistic effects of noradrenergic modulation with atomoxetine and 10 Hz repetitive transcranial magnetic stimulation on motor learning in healthy humans. BMC Neurosci. 2014; 15:46.9. Ueyama E, Ukai S, Ogawa A, Yamamoto M, Kawaguchi S, Ishii R, Shinosaki K. Chronic repetitive transcranial magnetic stimulation increases hippocampal neurogenesis in rats. Psychiatry Clin Neurosci. 2011; 65:77–81.10. Golmohammadi MG, Blackmore DG, Large B, Azari H, Esfandiary E, Paxinos G, Franklin KB, Reynolds BA, Rietze RL. Comparative analysis of the frequency and distribution of stem and progenitor cells in the adult mouse brain. Stem Cells. 2008; 26:979–987.11. Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011; 70:687–702.12. Feng SF, Shi TY, Fan Y, Wang WN, Chen YC, Tan QR. Long-lasting effects of chronic rTMS to treat chronic rodent model of depression. Behav Brain Res. 2012; 232:245–251.13. Guo F, Han X, Zhang J, Zhao X, Lou J, Chen H, Huang X. Repetitive transcranial magnetic stimulation promotes neural stem cell proliferation via the regulation of MiR-25 in a rat model of focal cerebral ischemia. PLoS One. 2014; 9:e109267.14. Azari H, Rahman M, Sharififar S, Reynolds BA. Isolation and expansion of the adult mouse neural stem cells using the neurosphere assay. J Vis Exp. 2010; (45):2393.15. Siebzehnrubl FA, Vedam-Mai V, Azari H, Reynolds BA, Deleyrolle LP. Isolation and characterization of adult neural stem cells. Methods Mol Biol. 2011; 750:61–77.16. Azari H, Sharififar S, Fortin JM, Reynolds BA. The neuroblast assay: an assay for the generation and enrichment of neuronal progenitor cells from differentiating neural stem cell progeny using flow cytometry. J Vis Exp. 2012; (62):3712.17. Azari H, Sharififar S, Darioosh RP, Fortin JM, Rahman M, Reynolds BA. Purifying immature neurons from differentiating neural stem cell progeny using a simple shaking method. J Stem Cell Res Ther. 2014; 4:178.18. Cho JM, Shin YJ, Park JM, Kim J, Lee MY. Characterization of nestin expression in astrocytes in the rat hippocampal CA1 region following transient forebrain ischemia. Anat Cell Biol. 2013; 46:131–140.19. Sachewsky N, Leeder R, Xu W, Rose KL, Yu F, van der Kooy D, Morshead CM. Primitive neural stem cells in the adult mammalian brain give rise to GFAP-expressing neural stem cells. Stem Cell Reports. 2014; 2:810–824.20. Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002; 8:963–970.21. Merlo S, Canonico PL, Sortino MA. Distinct effects of pramipexole on the proliferation of adult mouse sub-ventricular zone-derived cells and the appearance of a neuronal phenotype. Neuropharmacology. 2011; 60:892–900.22. Berg DA, Belnoue L, Song H, Simon A. Neurotransmitter-mediated control of neurogenesis in the adult vertebrate brain. Development. 2013; 140:2548–2561.23. Young SZ, Lafourcade CA, Platel JC, Lin TV, Bordey A. GABAergic striatal neurons project dendrites and axons into the postnatal subventricular zone leading to calcium activity. Front Cell Neurosci. 2014; 8:10.24. Daynac M, Chicheportiche A, Pineda JR, Gauthier LR, Boussin FD, Mouthon MA. Quiescent neural stem cells exit dormancy upon alteration of GABAAR signaling following radiation damage. Stem Cell Res. 2013; 11:516–528.25. Curtis MA, Penney EB, Pearson AG, van Roon-Mom WM, Butterworth NJ, Dragunow M, Connor B, Faull RL. Increased cell proliferation and neurogenesis in the adult human Huntington's disease brain. Proc Natl Acad Sci U S A. 2003; 100:9023–9027.26. Lennington JB, Pope S, Goodheart AE, Drozdowicz L, Daniels SB, Salamone JD, Conover JC. Midbrain dopamine neurons associated with reward processing innervate the neurogenic subventricular zone. J Neurosci. 2011; 31:13078–13087.27. Young SZ, Taylor MM, Bordey A. Neurotransmitters couple brain activity to subventricular zone neurogenesis. Eur J Neurosci. 2011; 33:1123–1132.28. Hitoshi S, Maruta N, Higashi M, Kumar A, Kato N, Ikenaka K. Antidepressant drugs reverse the loss of adult neural stem cells following chronic stress. J Neurosci Res. 2007; 85:3574–3585.29. Hoglinger GU, Rizk P, Muriel MP, Duyckaerts C, Oertel WH, Caille I, Hirsch EC. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci. 2004; 7:726–735.30. O'Keeffe GC, Tyers P, Aarsland D, Dalley JW, Barker RA, Caldwell MA. Dopamine-induced proliferation of adult neural precursor cells in the mammalian subventricular zone is mediated through EGF. Proc Natl Acad Sci U S A. 2009; 106:8754–8759.31. Sun LY, Hsieh DK, Yu TC, Chiu HT, Lu SF, Luo GH, Kuo TK, Lee OK, Chiou TW. Effect of pulsed electromagnetic field on the proliferation and differentiation potential of human bone marrow mesenchymal stem cells. Bioelectromagnetics. 2009; 30:251–260.32. Piacentini R, Ripoli C, Mezzogori D, Azzena GB, Grassi C. Extremely low-frequency electromagnetic fields promote in vitro neurogenesis via upregulation of Ca(v)1-channel activity. J Cell Physiol. 2008; 215:129–139.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Noninvasive brain stimulation: repetitive transcranial magnetic stimulation and transcranial direct current stimulation
- Repetitive Transcranial Magnetic Stimulation for Limb-Kinetic Apraxia in Parkinson's Disease
- Application of Non-invasive Brain Stimulation on Dysphagia after Stroke
- The Clinical Applications and the Electroencephalogram Effects of Repeated Transcranial Magnetic Stimulation
- Use of Repetitive Transcranial Magnetic Stimulation for Treatment in Psychiatry