J Korean Soc Radiol.
2014 Feb;70(2):123-132. 10.3348/jksr.2014.70.2.123.
Diagnostic Accuracy of Computed Tomography and Magnetic Resonance Imaging Obtained after Neoadjuvant Chemoradiotherapy in Predicting the Local Tumor Stage and Circumferential Resection Margin Status of Rectal Cancer
- Affiliations
-
- 1Department of Radiology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea. yhkrad@gmail.com
- 2Department of Radiology, Seoul National University Hospital, Seoul, Korea.
- 3Department of Surgery, Seoul National University Bundang Hospital, Seongnam, Korea.
- 4Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea.
- 5Department of Radiation Oncology, Seoul National University Bundang Hospital, Seongnam, Korea.
- 6Department of Pathology, Seoul National University Bundang Hospital, Seongnam, Korea.
- KMID: 1839421
- DOI: http://doi.org/10.3348/jksr.2014.70.2.123
Abstract
- PURPOSE
To measure the diagnostic accuracy of computed tomography (CT) and magnetic resonance imaging (MRI) obtained after neoadjuvant chemoradiotherapy (CRT) in patients with rectal cancer for a prediction of the local tumor stage and circumferential resection margin (CRM).
MATERIALS AND METHODS
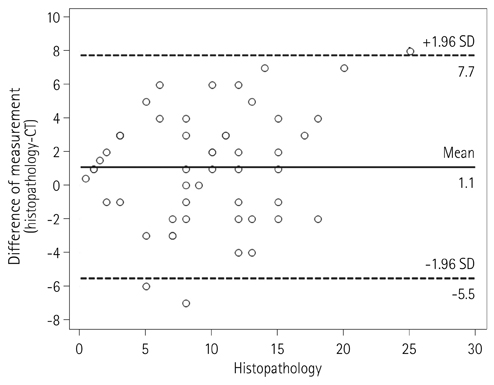
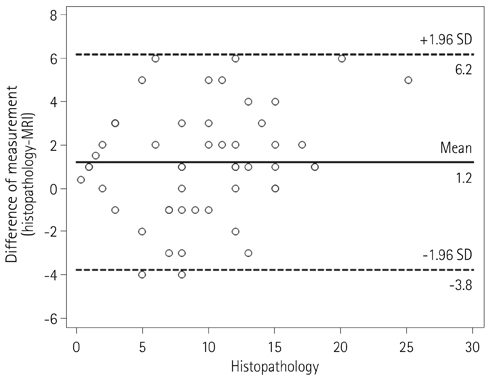
Two independent radiologists reviewed CT and MRI obtained after neoadjuvant CRT. The accuracy of the local tumor staging and the diagnostic performance for the prediction of CRM involvement were calculated. The agreement between the measurements of the distance to potential CRM on both imaging modalities and the histopathology findings was assessed using Bland-Altman plots.
RESULTS
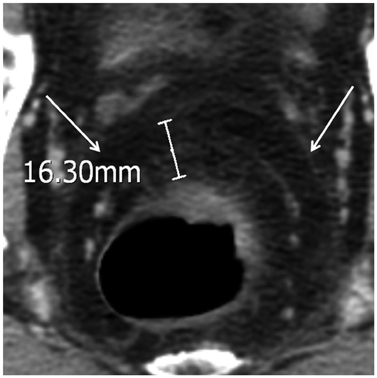
57 patients (mean age, 59.2 years; 24 females) were included. The accuracy of T and N staging were 43.9% (95% confidence interval, 30.8-57.7%) and 77.2% (64.2-87.3%) on CT and 63.2% (49.4-75.6%) and 77.2% (64.2-87.3%) on MRI for Observer 1. The accuracy of T and N staging were 54.4% (40.7-67.7%) and 77.2% (64.2-87.3%) on CT and 68.4% (54.7-80.1%) and 80.7% (68.1-90.0%) on MRI for Observer 2. Sensitivity and specificity on CRM involvement were 83.3% (43.7-97.0%) and 88.2% (76.6-94.5%) on CT and 100% (61.0-100%) and 90.2% (79.0-95.7%) on MRI for Observer 1. Sensitivity and specificity on CRM involvement were 66.7% (30.0-90.3%) and 88.2% (76.7-94.5%) on CT and 100% (61.0-100%) and 90.2% (79.0-95.7%) on MRI for Observer 2. Bland-Altman plots showed wide discrepancies between measurements of the distance to CRM on each CT and MRI and those on histopathology findings.
CONCLUSION
CT and MRI showed limited performance in predicting the local tumor staging and CRM involvement in patients with neoadjuvant CRT although MRI tended to show a better performance than CT
MeSH Terms
Figure
Reference
-
1. Adam IJ, Mohamdee MO, Martin IG, Scott N, Finan PJ, Johnston D, et al. Role of circumferential margin involvement in the local recurrence of rectal cancer. Lancet. 1994; 344:707–711.2. Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986; 1:1479–1482.3. García-Aguilar J, Hernandez de Anda E, Sirivongs P, Lee SH, Madoff RD, Rothenberger DA. A pathologic complete response to preoperative chemoradiation is associated with lower local recurrence and improved survival in rectal cancer patients treated by mesorectal excision. Dis Colon Rectum. 2003; 46:298–304.4. Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004; 351:1731–1740.5. Chen CC, Lee RC, Lin JK, Wang LW, Yang SH. How accurate is magnetic resonance imaging in restaging rectal cancer in patients receiving preoperative combined chemoradiotherapy? Dis Colon Rectum. 2005; 48:722–728.6. Kuo LJ, Chern MC, Tsou MH, Liu MC, Jian JJ, Chen CM, et al. Interpretation of magnetic resonance imaging for locally advanced rectal carcinoma after preoperative chemoradiation therapy. Dis Colon Rectum. 2005; 48:23–28.7. Suppiah A, Hunter IA, Cowley J, Garimella V, Cast J, Hartley JE, et al. Magnetic resonance imaging accuracy in assessing tumour down-staging following chemoradiation in rectal cancer. Colorectal Dis. 2009; 11:249–253.8. Kulkarni T, Gollins S, Maw A, Hobson P, Byrne R, Widdowson D. Magnetic resonance imaging in rectal cancer downstaged using neoadjuvant chemoradiation: accuracy of prediction of tumour stage and circumferential resection margin status. Colorectal Dis. 2008; 10:479–489.9. Pomerri F, Pucciarelli S, Maretto I, Zandonà M, Del Bianco P, Amadio L, et al. Prospective assessment of imaging after preoperative chemoradiotherapy for rectal cancer. Surgery. 2011; 149:56–64.10. Vliegen RF, Beets GL, Lammering G, Dresen RC, Rutten HJ, Kessels AG, et al. Mesorectal fascia invasion after neoadjuvant chemotherapy and radiation therapy for locally advanced rectal cancer: accuracy of MR imaging for prediction. Radiology. 2008; 246:454–462.11. Brown G, Daniels IR, Richardson C, Revell P, Peppercorn D, Bourne M. Techniques and trouble-shooting in high spatial resolution thin slice MRI for rectal cancer. Br J Radiol. 2005; 78:245–251.12. Greene FL, Page DL, Fleming ID, Fritz A, Balch CM, Haller DG. . AJCC cancer staging manual. 6th ed. New York: Springer-Verlag;2002.13. Brown G, Richards CJ, Newcombe RG, Dallimore NS, Radcliffe AG, Carey DP, et al. Rectal carcinoma: thin-section MR imaging for staging in 28 patients. Radiology. 1999; 211:215–222.14. Grubnic S, Vinnicombe SJ, Norman AR, Husband JE. MR evaluation of normal retroperitoneal and pelvic lymph nodes. Clin Radiol. 2002; 57:193–200. discussion 201-204.15. Beets-Tan RG, Beets GL, Vliegen RF, Kessels AG, Van Boven H, De Bruine A, et al. Accuracy of magnetic resonance imaging in prediction of tumour-free resection margin in rectal cancer surgery. Lancet. 2001; 357:497–504.16. Wieder HA, Rosenberg R, Lordick F, Geinitz H, Beer A, Becker K, et al. Rectal cancer: MR imaging before neoadjuvant chemotherapy and radiation therapy for prediction of tumor-free circumferential resection margins and long-term survival. Radiology. 2007; 243:744–751.17. Quirke P, Durdey P, Dixon MF, Williams NS. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision. Lancet. 1986; 2:996–999.18. Nagtegaal ID, Marijnen CA, Kranenbarg EK, van de Velde CJ, van Krieken JH. Pathology Review Committee. Cooperative Clinical Investigators. Circumferential margin involvement is still an important predictor of local recurrence in rectal carcinoma: not one millimeter but two millimeters is the limit. Am J Surg Pathol. 2002; 26:350–357.19. Arrivé L, Renard R, Carrat F, Belkacem A, Dahan H, Le Hir P, et al. A scale of methodological quality for clinical studies of radiologic examinations. Radiology. 2000; 217:69–74.20. Allen SD, Padhani AR, Dzik-Jurasz AS, Glynne-Jones R. Rectal carcinoma: MRI with histologic correlation before and after chemoradiation therapy. AJR Am J Roentgenol. 2007; 188:442–451.21. Kim JH, Beets GL, Kim MJ, Kessels AG, Beets-Tan RG. High-resolution MR imaging for nodal staging in rectal cancer: are there any criteria in addition to the size? Eur J Radiol. 2004; 52:78–83.22. Brown G, Richards CJ, Bourne MW, Newcombe RG, Radcliffe AG, Dallimore NS, et al. Morphologic predictors of lymph node status in rectal cancer with use of high-spatial-resolution MR imaging with histopathologic comparison. Radiology. 2003; 227:371–377.23. Lambregts DM, Cappendijk VC, Maas M, Beets GL, Beets-Tan RG. Value of MRI and diffusion-weighted MRI for the diagnosis of locally recurrent rectal cancer. Eur Radiol. 2011; 21:1250–1258.24. Beets-Tan RG, Lambregts DM, Maas M, Bipat S, Barbaro B, Caseiro-Alves F, et al. Magnetic resonance imaging for the clinical management of rectal cancer patients: recommendations from the 2012 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol. 2013; 23:2522–2531.25. Diop M, Parratte B, Tatu L, Vuillier F, Brunelle S, Monnier G. "Mesorectum" the surgical value of an anatomical approach. Surg Radiol Anat. 2003; 25:290–304.26. Garcia-Aguilar J, Smith DD, Avila K, Bergsland EK, Chu P, Krieg RM. Timing of Rectal Cancer Response to Chemoradiation Consortium. Optimal timing of surgery after chemoradiation for advanced rectal cancer: preliminary results of a multicenter, nonrandomized phase II prospective trial. Ann Surg. 2011; 254:97–102.27. Wang Y, Cummings B, Catton P, Dawson L, Kim J, Ringash J, et al. Primary radical external beam radiotherapy of rectal adenocarcinoma: long term outcome of 271 patients. Radiother Oncol. 2005; 77:126–132.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Significance of Preoperative Magnetic Resonance Imaging in Staging of Rectal Cancer
- Imaging Diagnosis of Locally Advanced Rectal Cancer: Tumor Staging before and after Preoperative Chemoradiotherapy
- Accuracy of Magnetic Resonance Imaging in Predicting TNM Staging and Circumferential Resection Margin Compared with Pathologic Assessment on Whole-mount Section in Rectal Cancer
- Circumferential Resection Margin Involvement in Stage III Rectal Cancer Patients Treated with Curative Resection Followed by Chemoradiotherapy: A Surrogate Marker for Local Recurrence?
- Preoperative Radiological Staging of Rectal Cancer