J Korean Med Sci.
2009 Feb;24(1):84-91. 10.3346/jkms.2009.24.1.84.
Regulation of Antiarrhythmic Drug Propafenone Effects on the C-type KV1.4 Potassium Channel by PHo and K+
- Affiliations
-
- 1Department of Cardiology, Zhongnan Hospital of Wuhan University, Wuhan, China. wangzhiquan1031@yahoo.com.cn
- 2Department of Physiology and Biophysics, University at Buffalo, SUNY, School of Medicine Biomedical Sciences, Buffalo, NY, USA.
- 3Department of Cardiology, Fuwai Hospital, Beijin, China.
- 4Department of Cardiology, Renmin Hospital of Wuhan University, Wuhan, China, China.
- 5Department of Cardiology, Dongfeng Hospital of Yunyang University, Shiyan, China.
- KMID: 1794411
- DOI: http://doi.org/10.3346/jkms.2009.24.1.84
Abstract
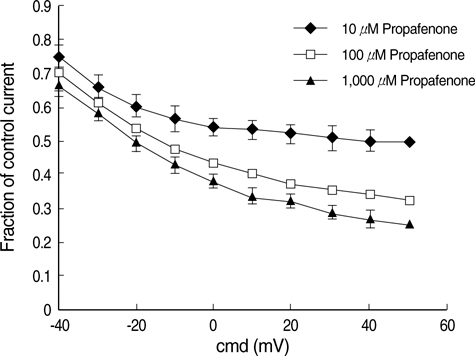
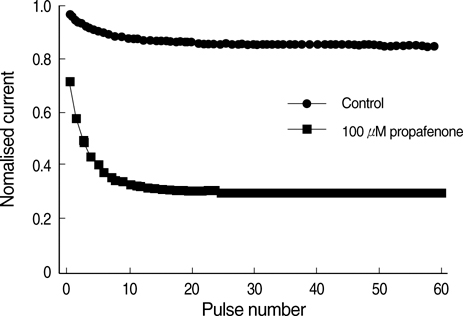
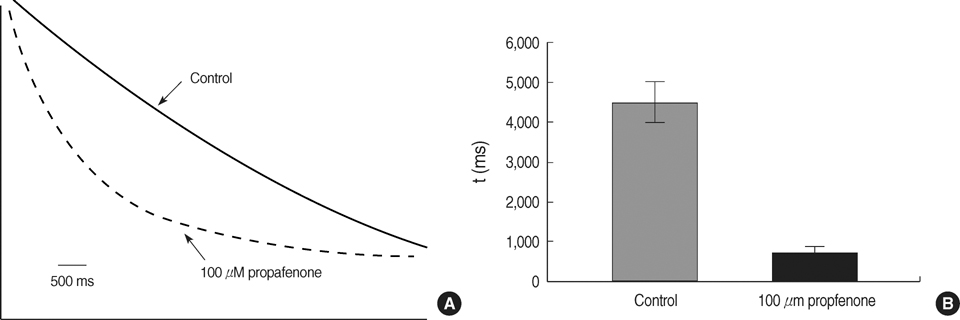
- The effects of the antiarrhythmic drug propafenone at c-type kv1.4 channels in Xenopus laevis oocytes were studied with the two-electrode voltage-clamp techinique. Defolliculated oocytes (stage V-VI) were injected with transcribed cRNAs of ferret Kv1.4 delta N channels. During recording, oocytes were continuously perfused with control solution or propafenone. Propafenone decreased the currents during voltage steps. The block was voltage-, use-, and concentration- dependent manners. The block was increased with positive going potentials. The voltage dependence of block could be fitted with the sum of monoexponential and a linear function. Propafenone accelerated the inactivate of current during the voltage step. The concentration of half-maximal block (IC(50)) was 121 micrometer/L. With high, normal, and low extracellular potassium concentrations, the changes of IC(50) value had no significant statistical differences. The block of propafenone was PH- dependent in high-, normal- and low- extracellular potassium concentrations. Acidification of the extracellular solution to PH 6.0 increased the IC50 values to 463 micrometer/L, alkalization to PH 8.0 reduced it to 58 micrometer/L. The results suggest that propafenone blocks the kv1.4 delta N channel in the open state and give some hints for an intracellular site of action.
Keyword
MeSH Terms
-
Animals
Anti-Arrhythmia Agents/*pharmacology
Hydrogen-Ion Concentration
Inhibitory Concentration 50
Kv1.4 Potassium Channel/*antagonists & inhibitors/metabolism
Oocytes/drug effects/metabolism
Patch-Clamp Techniques
Potassium/*metabolism
Potassium Channel Blockers/*pharmacology
Propafenone/*pharmacology
Xenopus laevis
Figure
Reference
-
1. Ledda F, Mantelli L, Manzini S, Amerini S, Mugelli A. Electrophysiological and antiarrhythmic properties of propafenon in isolated cardiac preparations. J Cardiovasc Pharmacol. 1981. 3:1162–1173.
Article2. Delgado C, Tamargo J, Tejerina T. Electrophysiological effects of propafenone in untreated and propafenone-pretreated guinea-pig atrial and ventricular muscles fibres. Br J Pharmacol. 1985. 86:765–775.3. Satoh H, Hashimoto K. Effect of propafenone on the membrane currents of rabbit sino-atrial node cells. Eur J Pharmacol. 1984. 99:185–191.
Article4. Duan D, Fermini B, Nattel S. Potassium channel blocking properties of propafenone in rabbit atrial myocytes. J Pharmacol Exp Ther. 1993. 264:1113–1123.5. Hoppe UC, Beuckelmann DJ. Modulation of the hyperpolarization-activated inward current (If) by antiarrhythmic agents in isolated human atrial myocytes. Naunyn Schmiedebergs Arch Pharmacol. 1998. 358:635–640.
Article6. Delpon E, Valenzuela C, Perez O, Casis O, Tamargo J. Propafenone preferentially blocks the rapidly activating component of delayed rectifier K+ current in guinea pig ventricular myocytes. Voltage-independent and time-dependent block of the slowly activating component. Circ Res. 1995. 76:223–235.7. Snyders DJ. Structure and function of cardiac potassium channels. Cardiovasc Res. 1999. 42:377–390.
Article8. Guo W, Kamiya K, Hojo M, Kodama I, Toyama J. Regualation of Kv1.4 K+ channel expression by myocardial hypertrophic factors in cultured newborn rat ventricular cells. J Mol Cell Cardiol. 1998. 30:1449–1455.9. Huang B, Qin D, El-Sherif N. Early down-regulation of K+ channel genes and currents in the postinfarction heart. J Cardiovasc Electrophysiol. 2000. 11:1252–1261.10. Matsubara H, Suzuki J, Inada M. Shaker-related potassium channel, Kv1.4, mRNA regulation in cultured rat heart myocytes and differential expression of Kv1.4 and Kv1.5 genes in myocardial development and hypertrophy. J Clin Invest. 1993. 92:1659–1666.
Article11. Nishiyama A, Ishii DN, Backx PH, Pulford BE, Birks BR, Tamkun MM. Altered K+ channel gene expression in diabetic rat ventricle: isoform switching between Kv4.2 and Kv1.4. Am J Physiol Heart Circ Physiol. 2001. 281:H1800–H1807.12. Comer MB, Campbell DL, Rasmusson RL, Lamson DR, Morales MJ, Zhang Y, Strauss HC. Cloning and characterization of an Ito-like potassium channel from ferret ventricle. Am J Physiol. 1994. 267:H1383–H1395.
Article13. Rasmusson RL, Morales MJ, Castellino RC, Zhang Y, Campbell DL, Strauss HC. C-type inactivation controls recovery in a fast inactivating cardiac K+ channel (Kv1.4) expressed in Xenopus oocytes. J Physiol. 1995. 489:709–721.14. Madeja M, Musshoff U, Speckmann EJ. A concentration-clamp system allowing two-electrode voltage-clamp investigations in oocytes of Xenopus laevis. J Neurosci Methods. 1991. 38:267–269.
Article15. Franqueza L, Valenzuela C, Delpon E, Longobardo M, Caballero R, Tamargo J. Effects of propafenone and 5-hydroxy-propafenone on hKv1.5 channels. Br J Pharmacol. 1998. 125:969–978.
Article16. Mergenthaler J, Haverkamp W, Huttenhofer A, Skryabin BV, Musshoff U, Borggrefe M, Speckmann EJ, Breithsrdt G, Madeja M. Blocking effects of the antiarrhythmic drug propafenone on the HERG potassium channel. Naunyn Schmiedebergs Arch Pharmacol. 2001. 363:472–480.
Article17. Baukrowitz T, Yellen G. Modulation of K+ current by frequency and external [K+]: a tale of two inactivation mechanisms. Neuron. 1995. 15:951–960.18. Wang S, Liu S, Morales MJ, Strauss HC, Rasmusson RL. A quantitative analysis of the activation and inactivation kinetics of HERG expressed in Xenopus oocytes. J Physiol. 1997. 502:45–60.19. Ficker E, Jarolimek J, Kiehn J, Baumann A, Brown AM. Molecular determinants of dofetilide block of HERG K+ channels. Circ Res. 1998. 82:386–395.20. Rasmusson RL, Morales MJ, Wang S, Liu S, Campbell DL, Brahmajothi MV, Strauss HC. Inactivation of voltage-gated cardiac K+ channels. Circ Res. 1998. 82:739–750.21. Li X, Bett GC, Jiang X, Bondarenko VE, Morales MJ, Rasmusson RL. Regulation of N-and C-type inactivation of Kv1.4 by pHo and K+: evidence for transmembrane communication. Am J Physiol Heart Circ Physiol. 2003. 284:H71–H80.22. Wang SY, Wang GK. A mutation in segment I-S6 alters slow inactivation of sodium channels. Biophys J. 1997. 72:1633–1640.
Article23. Chen J, Seebohm G, Sanguinetti MC. Position of aromatic residues in the S6 domain, not inactivation, dictates cisapride sensitivity of HERG and eag potassium channels. Proc Natl Acad Sci USA. 2002. 99:12461–12466.
Article24. Madeja M, Leicher T, Friederich P, Punke MA, Haverkamp W, Musshoff U, Breithardt G, Speckmann EJ. Molecular site of action of the antiarrhythmic drug propafenone at the voltage-operated potassium channel Kv2.1. Mol Pharmacol. 2003. 63:547–556.
Article25. Kobertz WR, Williams C, Miller C. Hanging gondola structure of the T1 domain in a voltage-gated K(+) channel. Biochemistry. 2000. 39:10347–10352.26. Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998. 280:69–77.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibition of voltage-dependent K⺠current in rabbit coronary arterial smooth muscle cells by the class Ic antiarrhythmic drug propafenone
- The antidiabetic drug rosiglitazone blocks Kv1.5 potassium channels in an open state
- Fluoxetine Protects against Big Endothelin-1 Induced Anti-Apoptosis by Rescuing Kv1.5 Channels in Human Pulmonary Arterial Smooth Muscle Cells
- Effects of 3,3′,4,4′,5-pentachlorobiphenyl on human Kv1.3 and Kv1.5 channels
- Wide QRS tachycardia during treatment with class ic antiarrhythmic agents in a patient with atrial fibrillation: report of 2 cases