Yonsei Med J.
2012 Jul;53(4):842-848. 10.3349/ymj.2012.53.4.842.
Fluoxetine Protects against Big Endothelin-1 Induced Anti-Apoptosis by Rescuing Kv1.5 Channels in Human Pulmonary Arterial Smooth Muscle Cells
- Affiliations
-
- 1Department of Cardiothoracic Surgery, Renmin Hospital of Wuhan University, Wuhan, China. zfmao2007@163.com
- KMID: 1716887
- DOI: http://doi.org/10.3349/ymj.2012.53.4.842
Abstract
- PURPOSE
Pulmonary Kv channels are thought to play a crucial role in the regulation of cell proliferation and apoptosis. Previous studies have shown that fluoxetine upregulated the expression of Kv1.5 and prevented pulmonary arterial hypertension in monocrotaline-induced or hypoxia-induced rats and mice. The current study was designed to test how fluoxetine regulates Kv1.5 channels, subsequently promoting apoptosis in human PASMCs cultured in vitro.
MATERIALS AND METHODS
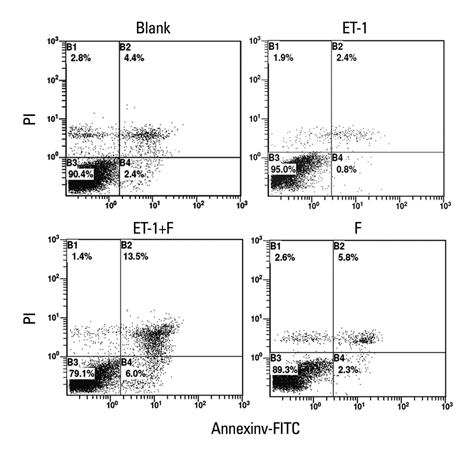
Human PASMCs were incubated with low-serum DMEM, ET-1, and fluoxetine with and without ET-1 separately for 72 h. Then the proliferation, apoptosis, and expression of TRPC1 and Kv1.5 were detected.
RESULTS
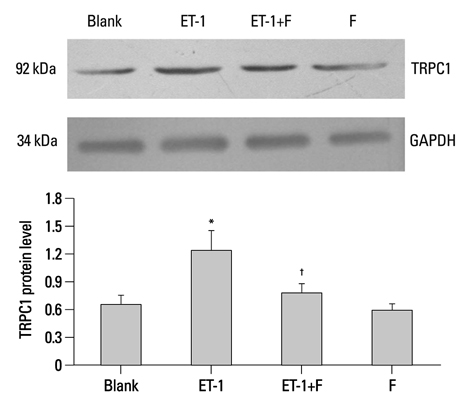
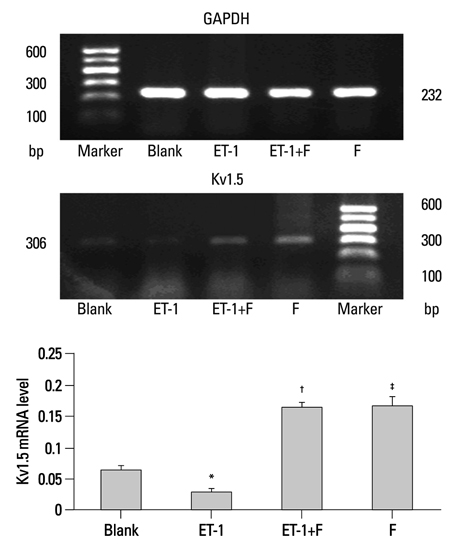
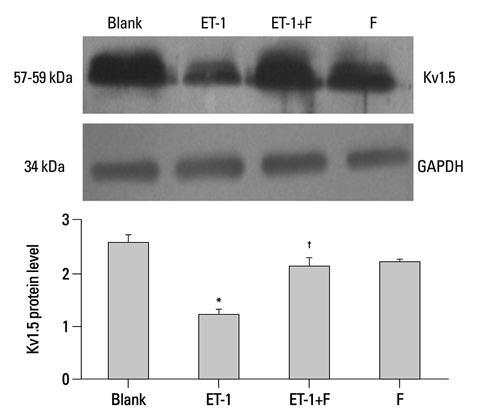
In the ET-1 induced group, the upregulation of TRPC1 and down regulation of Kv1.5 enhanced proliferation and anti-apoptosis, which was reversed when treated with fluoxetine. The decreased expression of TRPC1 increased the expression of Kv1.5, subsequently inhibiting proliferation while promoting apoptosis.
CONCLUSION
The results from the present study suggested that fluoxetine protects against big endothelin-1 induced anti-apoptosis and rescues Kv1.5 channels in human pulmonary arterial smooth muscle cells, potentially by decreasing intracellular concentrations of Ca2+.
MeSH Terms
-
Apoptosis/drug effects/genetics
Blotting, Western
Cell Proliferation/drug effects
Cells, Cultured
Endothelin-1/*pharmacology
Flow Cytometry
Fluoxetine/*pharmacology
Humans
Kv1.5 Potassium Channel/genetics/*metabolism
Muscle, Smooth, Vascular/*cytology/drug effects
Pulmonary Artery/*cytology
Reverse Transcriptase Polymerase Chain Reaction
Figure
Reference
-
1. Bonnet S, Rochefort G, Sutendra G, Archer SL, Haromy A, Webster L, et al. The nuclear factor of activated T cells in pulmonary arterial hypertension can be therapeutically targeted. Proc Natl Acad Sci U S A. 2007. 104:11418–11423.
Article2. Remillard CV, Tigno DD, Platoshyn O, Burg ED, Brevnova EE, Conger D, et al. Function of Kv1.5 channels and genetic variations of KCNA5 in patients with idiopathic pulmonary arterial hypertension. Am J Physiol Cell Physiol. 2007. 292:C1837–C1853.3. Bonnet S, Michelakis ED, Porter CJ, Andrade-Navarro MA, Thébaud B, Bonnet S, et al. An abnormal mitochondrial-hypoxia inducible factor-1alpha-Kv channel pathway disrupts oxygen sensing and triggers pulmonary arterial hypertension in fawn hooded rats: similarities to human pulmonary arterial hypertension. Circulation. 2006. 113:2630–2641.
Article4. McMurtry MS, Bonnet S, Wu X, Dyck JR, Haromy A, Hashimoto K, et al. Dichloroacetate prevents and reverses pulmonary hypertension by inducing pulmonary artery smooth muscle cell apoptosis. Circ Res. 2004. 95:830–840.
Article5. Michel RP, Langleben D, Dupuis J. The endothelin system in pulmonary hypertension. Can J Physiol Pharmacol. 2003. 81:542–554.
Article6. Rubens C, Ewert R, Halank M, Wensel R, Orzechowski HD, Schultheiss HP, et al. Big endothelin-1 and endothelin-1 plasma levels are correlated with the severity of primary pulmonary hypertension. Chest. 2001. 120:1562–1569.
Article7. Shimoda LA, Sylvester JT, Booth GM, Shimoda TH, Meeker S, Undem BJ, et al. Inhibition of voltage-gated K(+) currents by endothelin-1 in human pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol. 2001. 281:L1115–L1122.8. Marcos E, Adnot S, Pham MH, Nosjean A, Raffestin B, Hamon M, et al. Serotonin transporter inhibitors protect against hypoxic pulmonary hypertension. Am J Respir Crit Care Med. 2003. 168:487–493.
Article9. Guignabert C, Raffestin B, Benferhat R, Raoul W, Zadigue P, Rideau D, et al. Serotonin transporter inhibition prevents and reverses monocrotaline-induced pulmonary hypertension in rats. Circulation. 2005. 111:2812–2819.
Article10. Zhu SP, Mao ZF, Huang J, Wang JY. Continuous fluoxetine administration prevents recurrence of pulmonary arterial hypertension and prolongs survival in rats. Clin Exp Pharmacol Physiol. 2009. 36:e1–e5.
Article11. Zhai FG, Zhang XH, Wang HL. Fluoxetine protects against monocrotaline-induced pulmonary arterial hypertension: potential roles of induction of apoptosis and upregulation of Kv1.5 channels in rats. Clin Exp Pharmacol Physiol. 2009. 36:850–856.
Article12. Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med. 2004. 351:1655–1665.
Article13. Fantozzi I, Platoshyn O, Wong AH, Zhang S, Remillard CV, Furtado MR, et al. Bone morphogenetic protein-2 upregulates expression and function of voltage-gated K+ channels in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2006. 291:L993–L1004.14. Krick S, Platoshyn O, McDaniel SS, Rubin LJ, Yuan JX. Augmented K(+) currents and mitochondrial membrane depolarization in pulmonary artery myocyte apoptosis. Am J Physiol Lung Cell Mol Physiol. 2001. 281:L887–L894.15. Burg ED, Remillard CV, Yuan JX. Potassium channels in the regulation of pulmonary artery smooth muscle cell proliferation and apoptosis: pharmacotherapeutic implications. Br J Pharmacol. 2008. 153:Suppl 1. S99–S111.
Article16. Platoshyn O, Golovina VA, Bailey CL, Limsuwan A, Krick S, Juhaszova M, et al. Sustained membrane depolarization and pulmonary artery smooth muscle cell proliferation. Am J Physiol Cell Physiol. 2000. 279:C1540–C1549.
Article17. Konduri GG, Bakhutashvili I, Eis A, Gauthier KM. Impaired voltage gated potassium channel responses in a fetal lamb model of persistent pulmonary hypertension of the newborn. Pediatr Res. 2009. 66:289–294.
Article18. Neylon CB. Potassium channels and vascular proliferation. Vascul Pharmacol. 2002. 38:35–41.
Article19. Post JM, Gelband CH, Hume JR. [Ca2+]i inhibition of K+ channels in canine pulmonary artery. Novel mechanism for hypoxia-induced membrane depolarization. Circ Res. 1995. 77:131–139.20. Wang C, Wang J, Zhao L, Wang Y, Liu J, Shi L, et al. Sildenafil inhibits human pulmonary artery smooth muscle cell proliferation by decreasing capacitative Ca2+ entry. J Pharmacol Sci. 2008. 108:71–78.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Fluoxetine on Potassium Channels in Cat Gastric Smooth Muscle Cells
- Change of voltage-gated potassium channel 1.7 expressions in monocrotaline-induced pulmonary arterial hypertension rat model
- Effects of 3,3′,4,4′,5-pentachlorobiphenyl on human Kv1.3 and Kv1.5 channels
- Pathophysiology of Persistent Pulmonary Hypertension of the Newborn
- Changes of Endothelin-1 Level after the Closure of Patent Ductus Arteriosus