Clinical Features and Mutations in the ENG, ACVRL1, and SMAD4 genes in Korean Patients with Hereditary Hemorrhagic Telangiectasia
- Affiliations
-
- 1Department of Laboratory Medicine and Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. changski@skku.edu
- 2Garak High School, Seoul, Korea.
- 3Department of Internal Medicine, Cardiac and Vascular Center, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 4Department of Radiology, Samsung Medical Center, Sungkyunkwan University, School of Medicine, Seoul, Korea.
- 5Division of Pulmonary and Critical Care Medicine, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 1794409
- DOI: http://doi.org/10.3346/jkms.2009.24.1.69
Abstract
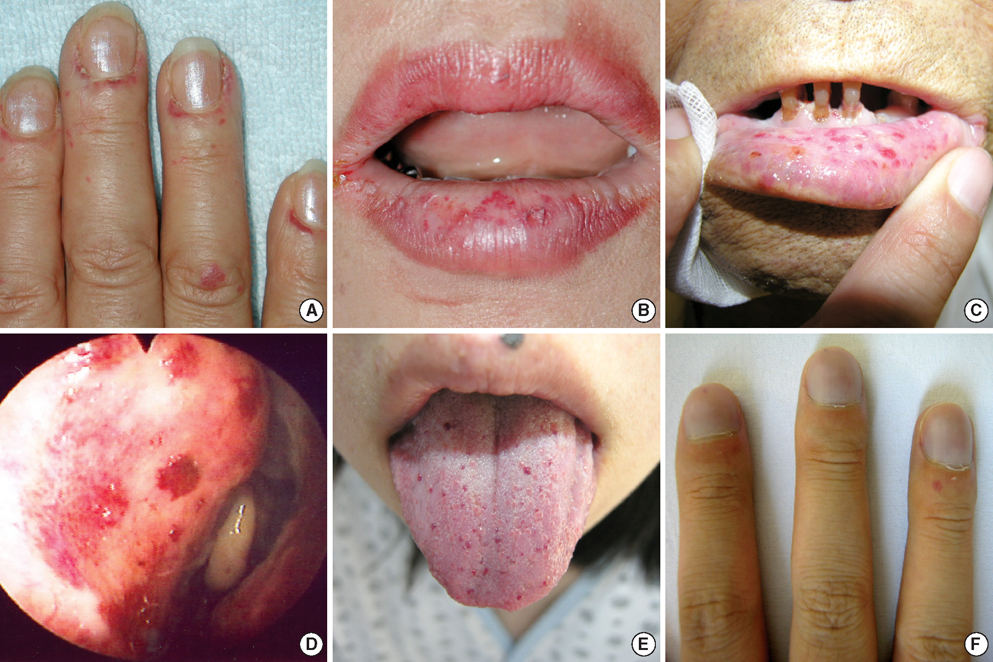
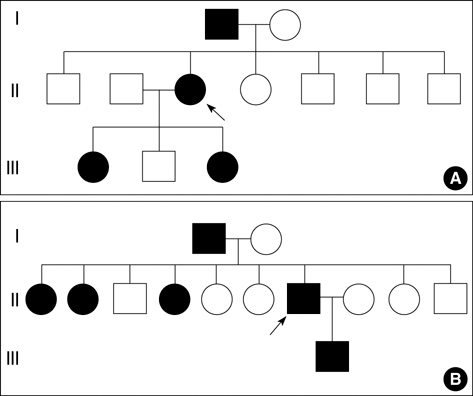
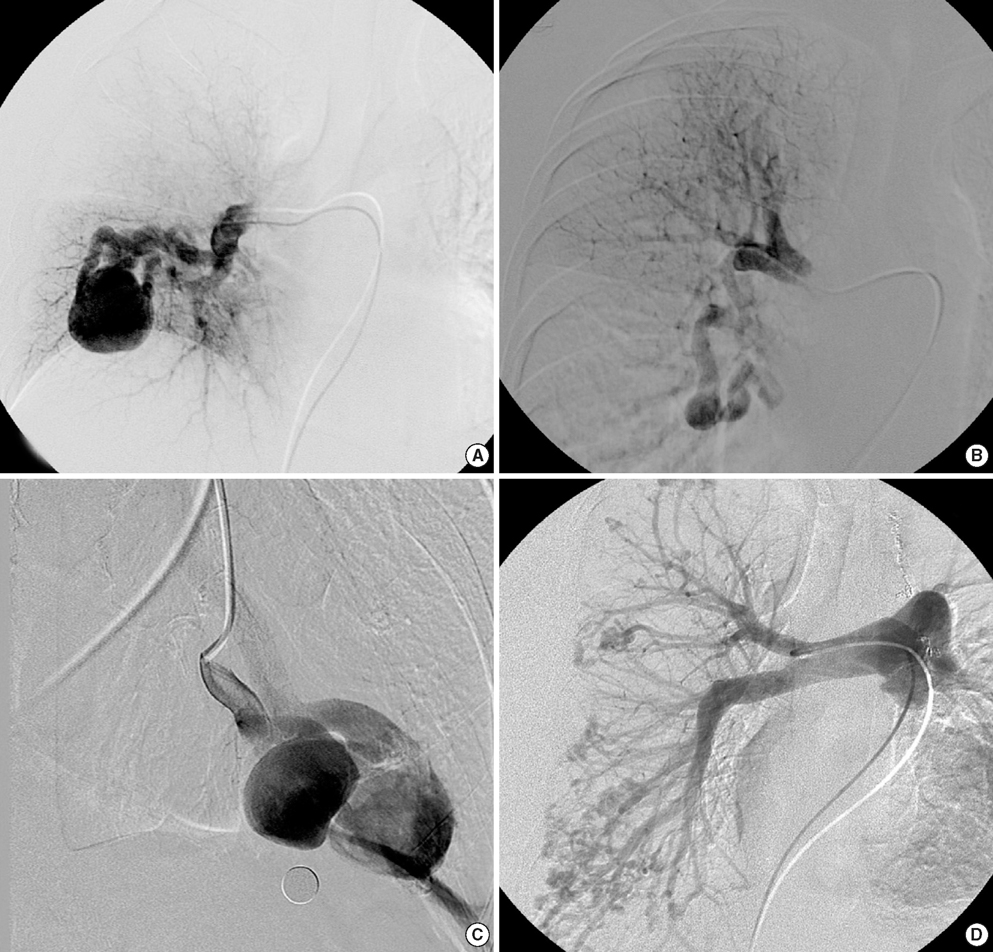
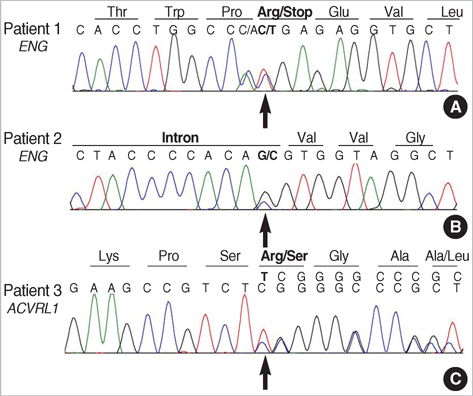
- Hereditary hemorrhagic telangiectasia (HHT) is an inherited disorder that is characterized by abnormal communication between the arteries and veins in the skin, mucosa, and various organs. HHT has been reported to show significant phenotypic variability and genetic heterogeneity with wide ethnic and geographic variations. Although mutations in the endoglin (ENG) and activin A receptor type II-like 1 (ACVRL1) genes have been known to cause HHT for more than 10 yr, little is known about the clinical features or genetic background of Korean patients with HHT. In addition, mutations in mothers against decapentaplegic homolog 4 (SMAD4) are also seen in patients with the combined syndrome of juvenile polyposis and HHT. This study examined five Korean patients with the typical manifestations of HHT such as frequent epistaxis and pulmonary arteriovenous malformations. Direct sequencing of the ENG and ACVRL1 genes revealed one known mutation, ENG c.277C>T, in one patient and two novel mutations, ENG c.992-1G>C and ACVRL1 c.81dupT in two patients, respectively. The remaining two patients with negative results were screened for SMAD4 mutations as well as gross deletions of ENG and ACVRL1 using multiple ligation-dependent probe amplification, but none was detected. Despite the small number of patients investigated, we firstly report Korean patients with genetically confirmed HHT, and show the genetic and allelic heterogeneity underlying HHT.
MeSH Terms
-
Activin Receptors, Type II/*genetics
Adult
Alleles
Angiography
Antigens, CD/*genetics
Asian Continental Ancestry Group/*genetics
Base Sequence
Female
Genetic Predisposition to Disease
Humans
Korea
Male
Middle Aged
*Mutation
Pedigree
Receptors, Cell Surface/*genetics
Smad4 Protein/*genetics
Telangiectasia, Hereditary Hemorrhagic/diagnosis/*genetics/pathology
Tomography, X-Ray Computed
Young Adult
Figure
Cited by 2 articles
-
Spectrum of Novel Hereditary Hemorrhagic Telangiectasia Variants in an Austrian Patient Cohort
Martin Koenighofer, Thomas Parzefall, Alexandra Frohne, Matthew Allen, Ursula Unterberger, Franco Laccone, Christian Schoefer, Klemens Frei, Trevor Lucas
Clin Exp Otorhinolaryngol. 2019;12(4):405-411. doi: 10.21053/ceo.2019.00304.Genetic Variants and Clinical Phenotypes in Korean Patients With Hereditary Hemorrhagic Telangiectasia
Bo-Gyeong Kim, Joo-hyun Jung, Mi-Jung Kim, Eun-Hye Moon, Jae-Hwan Oh, Jung-Woo Park, Heung-Eog Cha, Ju-Hyun Kim, Yoon-Jae Kim, Jun-Won Chung, Ki-Baik Hahm, Hong-Ryul Jin, Yong-Ju Jang, Sung Wan Kim, Seung-Kyu Chung, Dae-Woo Kim, Young Jae Lee, Seon-Tae Kim
Clin Exp Otorhinolaryngol. 2021;14(4):399-406. doi: 10.21053/ceo.2020.02124.
Reference
-
1. Peery WH. Clinical spectrum of hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu disease). Am J Med. 1987. 82:989–997.
Article2. Abdalla SA, Letarte M. Hereditary haemorrhagic telangiectasia: current views on genetics and mechanisms of disease. J Med Genet. 2006. 43:97–110.
Article3. Westermann CJ, Rosina AF, De Vries V, de Coteau PA. The prevalence and manifestations of hereditary hemorrhagic telangiectasia in the Afro-Caribbean population of the Netherlands Antilles: a family screening. Am J Med Genet A. 2003. 116:324–328.
Article4. Porteous ME, Burn J, Proctor SJ. Hereditary haemorrhagic telangiectasia: a clinical analysis. J Med Genet. 1992. 29:527–530.
Article5. Guttmacher AE, Marchuk DA, White RI Jr. Hereditary hemorrhagic telangiectasia. N Engl J Med. 1995. 333:918–924.
Article6. Dakeishi M, Shioya T, Wada Y, Shindo T, Otaka K, Manabe M, Nozaki J, Inoue S, Koizumi A. Genetic epidemiology of hereditary hemorrhagic telangiectasia in a local community in the northern part of Japan. Hum Mutat. 2002. 19:140–148.
Article7. Kjeldsen AD, Vase P, Green A. Hereditary haemorrhagic telangiectasia: a population-based study of prevalence and mortality in Danish patients. J Intern Med. 1999. 245:31–39.
Article8. Bideau A, Plauchu H, Jacquard A, Robert JM, Desjardins B. Genetic aspects of Rendu-Osler disease in Haut-Jura: convergence of methodological approaches of historic demography and medical genetics. J Genet Hum. 1980. 28:127–147.9. Plauchu H, de Chadarevian JP, Bideau A, Robert JM. Age-related clinical profile of hereditary hemorrhagic telangiectasia in an epidemiologically recruited population. Am J Med Genet. 1989. 32:291–297.
Article10. Shovlin CL, Guttmacher AE, Buscarini E, Faughnan ME, Hyland RH, Westermann CJ, Kjeldsen AD, Plauchu H. Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber syndrome). Am J Med Genet. 2000. 91:66–67.
Article11. McAllister KA, Grogg KM, Johnson DW, Gallione CJ, Baldwin MA, Jackson CE, Helmbold EA, Markel DS, McKinnon WC, Murrell J, McCormick MK, Pericak-Vance MA, Heutink P, Oostra BA, Haitjema T, Westerman CJ, Porteous ME, Guttmacher AE, Letarte M, Marchuk DA. Endoglin, a TGF-beta binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat Genet. 1994. 8:345–351.12. Johnson DW, Berg JN, Gallione CJ, McAllister KA, Warner JP, Helmbold EA, Markel DS, Jackson CE, Porteous ME, Marchuk DA. A second locus for hereditary hemorrhagic telangiectasia maps to chromosome 12. Genome Res. 1995. 5:21–28.
Article13. Bayrak-Toydemir P, McDonald J, Markewitz B, Lewin S, Miller F, Chou LS, Gedge F, Tang W, Coon H, Mao R. Genotype-phenotype correlation in hereditary hemorrhagic telangiectasia: mutations and manifestations. Am J Med Genet A. 2006. 140:463–470.
Article14. Bossler AD, Richards J, George C, Godmilow L, Ganguly A. Novel mutations in ENG and ACVRL1 identified in a series of 200 individuals undergoing clinical genetic testing for hereditary hemorrhagic telangiectasia (HHT): correlation of genotype with phenotype. Hum Mutat. 2006. 27:667–675.15. Lesca G, Olivieri C, Burnichon N, Pagella F, Carette MF, Gilbert-Dussardier B, Goizet C, Roume J, Rabilloud M, Saurin JC, Cottin V, Honnorat J, Coulet F, Giraud S, Calender A, Danesino C, Buscarini E, Plauchu H. Genotype-phenotype correlations in hereditary hemorrhagic telangiectasia: data from the French-Italian HHT network. Genet Med. 2007. 9:14–22.
Article16. Letteboer TG, Mager JJ, Snijder RJ, Koeleman BP, Lindhout D, Ploos van Amstel JK, Westermann CJ. Genotype-phenotype relationship in hereditary haemorrhagic telangiectasia. J Med Genet. 2006. 43:371–377.17. Berg J, Porteous M, Reinhardt D, Gallione C, Holloway S, Umasunthar T, Lux A, McKinnon W, Marchuk D, Guttmacher A. Hereditary haemorrhagic telangiectasia: a questionnaire based study to delineate the different phenotypes caused by endoglin and ALK1 mutations. J Med Genet. 2003. 40:585–590.
Article18. Pece N, Vera S, Cymerman U, White RI Jr, Wrana JL, Letarte M. Mutant endoglin in hereditary hemorrhagic telangiectasia type 1 is transiently expressed intracellularly and is not a dominant negative. J Clin Invest. 1997. 100:2568–2579.
Article19. Cole SG, Begbie ME, Wallace GM, Shovlin CL. A new locus for hereditary haemorrhagic telangiectasia (HHT3) maps to chromosome 5. J Med Genet. 2005. 42:577–582.
Article20. Bayrak-Toydemir P, McDonald J, Akarsu N, Toydemir RM, Calderon F, Tuncali T, Tang W, Miller F, Mao R. A fourth locus for hereditary hemorrhagic telangiectasia maps to chromosome 7. Am J Med Genet A. 2006. 140:2155–2162.
Article21. Gallione CJ, Repetto GM, Legius E, Rustgi AK, Schelley SL, Tejpar S, Mitchell G, Drouin E, Westermann CJ, Marchuk DA. A combined syndrome of juvenile polyposis and hereditary haemorrhagic telangiectasia associated with mutations in MADH4 (SMAD4). Lancet. 2004. 363:852–859.22. Bergler W, Sadick H, Gotte K, Riedel F, Hormann K. Topical estrogens combined with argon plasma coagulation in the management of epistaxis in hereditary hemorrhagic telangiectasia. Ann Otol Rhinol Laryngol. 2002. 111(3 Pt 1):222–228.
Article23. Cymerman U, Vera S, Pece-Barbara N, Bourdeau A, White RI Jr, Dunn J, Letarte M. Identification of hereditary hemorrhagic telangiectasia type 1 in newborns by protein expression and mutation analysis of endoglin. Pediatr Res. 2000. 47:24–35.
Article24. Haitjema T, Balder W, Disch FJ, Westermann CJ. Epistaxis in hereditary haemorrhagic telangiectasia. Rhinology. 1996. 34:176–178.25. Sadick H, Sadick M, Gotte K, Naim R, Riedel F, Bran G, Hormann K. Hereditary hemorrhagic telangiectasia: an update on clinical manifestations and diagnostic measures. Wien Klin Wochenschr. 2006. 118:72–80.
Article26. Sabba C. A rare and misdiagnosed bleeding disorder: hereditary hemorrhagic telangiectasia. J Thromb Haemost. 2005. 3:2201–2210.27. Cottin V, Chinet T, Lavole A, Corre R, Marchand E, Reynaud-Gaubert M, Plauchu H, Cordier JF. Pulmonary arteriovenous malformations in hereditary hemorrhagic telangiectasia: a series of 126 patients. Medicine (Baltimore). 2007. 86:1–17.28. KoreaMed. Korean Association of Medical Journal Editors. Available at http://www.koreamed.org.29. Butter A, Emran M, Al-Jazaeri A, Bouron-Dal Soglio D, Bouchard S. Pulmonary arteriovenous malformation mimicking congenital cystic adenomatoid malformation in a newborn. J Pediatr Surg. 2006. 41:e9–e11.30. Llorca O, Trujillo A, Blanco FJ, Bernabeu C. Structural model of human endoglin, a transmembrane receptor responsible for hereditary hemorrhagic telangiectasia. J Mol Biol. 2007. 365:694–705.
Article31. ten Dijke P, Ichijo H, Franzen P, Schulz P, Saras J, Toyoshima H, Heldin CH, Miyazono K. Activin receptor-like kinases: a novel subclass of cell-surface receptors with predicted serine/threonine kinase activity. Oncogene. 1993. 8:2879–2887.32. Seki T, Yun J, Oh SP. Arterial endothelium-specific activin receptor-like kinase 1 expression suggests its role in arterialization and vascular remodeling. Circ Res. 2003. 93:682–689.
Article33. Lesca G, Plauchu H, Coulet F, Lefebvre S, Plessis G, Odent S, Riviere S, Leheup B, Goizet C, Carette MF, Cordier JF, Pinson S, Soubrier F, Calender A, Giraud S. Molecular screening of ALK1/ACVRL1 and ENG genes in hereditary hemorrhagic telangiectasia in France. Hum Mutat. 2004. 23:289–299.34. Schulte C, Geisthoff U, Lux A, Kupka S, Zenner HP, Blin N, Pfister M. High frequency of ENG and ALK1/ACVRL1 mutations in German HHT patients. Hum Mutat. 2005. 25:595.
Article35. Cymerman U, Vera S, Karabegovic A, Abdalla S, Letarte M. Characterization of 17 novel endoglin mutations associated with hereditary hemorrhagic telangiectasia. Hum Mutat. 2003. 21:482–492.
Article36. Gallione CJ, Richards JA, Letteboer TG, Rushlow D, Prigoda NL, Leedom TP, Ganguly A, Castells A, Ploos van Amstel JK, Westermann CJ, Pyeritz RE, Marchuk DA. SMAD4 mutations found in unselected HHT patients. J Med Genet. 2006. 43:793–797.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current Status of Clinical Diagnosis and Genetic Analysis of Hereditary Hemorrhagic Telangiectasia in South Korea: Multicenter Case Series and a Systematic Review
- Spectrum of Novel Hereditary Hemorrhagic Telangiectasia Variants in an Austrian Patient Cohort
- Genetic Variants and Clinical Phenotypes in Korean Patients With Hereditary Hemorrhagic Telangiectasia
- Hereditary Hemorrhagic Telangiectasia in a Family
- Hereditary Hemorrhagic Telangiectasia Combined with Pulmonary Arteriovenous Malformation Treated with Transcatheter Embolotherapy