Clin Exp Otorhinolaryngol.
2019 Nov;12(4):405-411. 10.21053/ceo.2019.00304.
Spectrum of Novel Hereditary Hemorrhagic Telangiectasia Variants in an Austrian Patient Cohort
- Affiliations
-
- 1Department of Otorhinolaryngology, Head and Neck Surgery, Medical University of Vienna, Vienna, Austria. klemens.frei@meduniwien.ac.at
- 2Center for Anatomy and Cell Biology, Department for Cell and Developmental Biology, Medical University of Vienna, Vienna, Austria.
- 3Institute of Medical Genetics, Medical University of Vienna, Vienna, Austria.
- KMID: 2462737
- DOI: http://doi.org/10.21053/ceo.2019.00304
Abstract
OBJECTIVES
Hereditary hemorrhagic telangiectasia (HHT) is a rare autosomal dominant genetic disorder characterized by pathogenic blood vessel development and maintenance. HHT type 1 (HHT1) and type 2 (HHT2) are caused by variants in endoglin (ENG) and activin receptor-like kinase-1 (ACVRL1), respectively. The aim of this study was to identify the spectrum of pathogenic variants in ENG and ACVRL1 in Austrian HHT families.
METHODS
In this prospective study, eight Austrian HHT families were screened for variants in ENG and ACVRL1 by polymerase chain reaction amplification and sequencing of DNA isolated from peripheral blood.
RESULTS
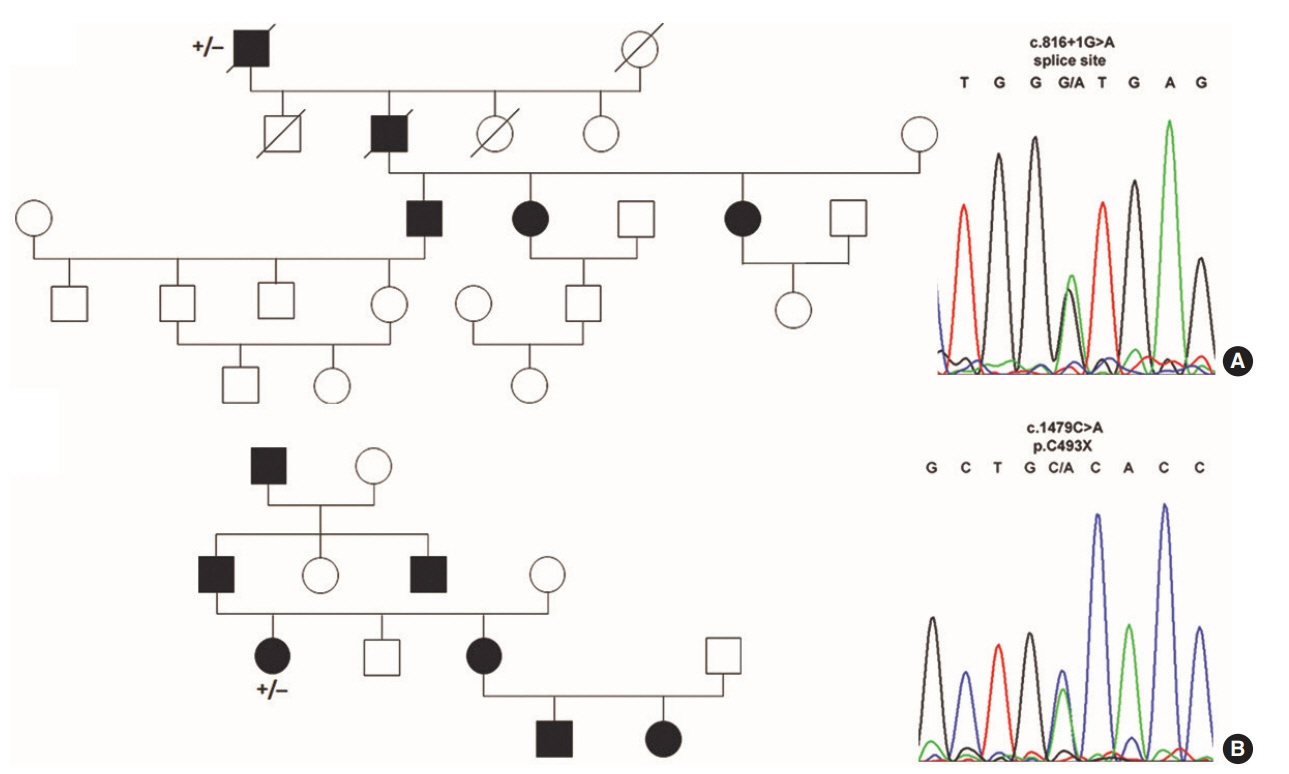
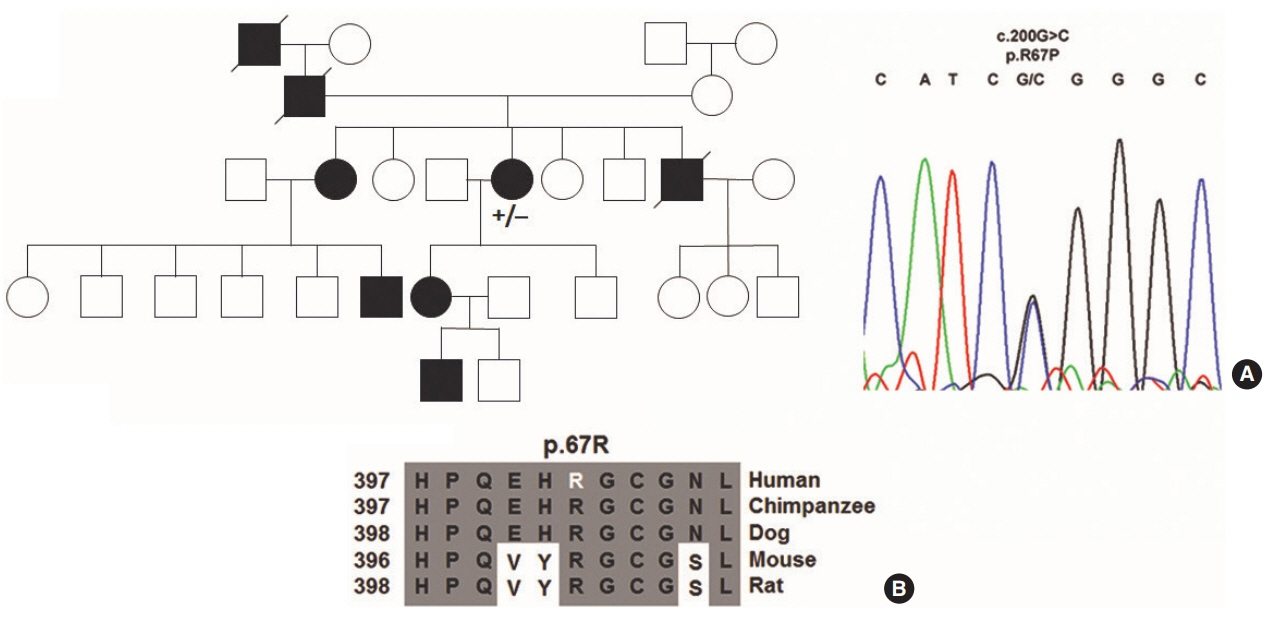
Heterozygous variants were identified in all families under study. HHT1 was caused by a novel c.816+1G>A splice donor variant, a novel c.1479C>A nonsense (p.Cys493X) variant and a published c.1306C>T nonsense (p.Gln436X) variant in ENG. Variants found in ACVRL1 were novel c.200G>C (p.Arg67Pro) and known c.772G>A (p.Gly258Ser) missense variants in highly conserved residues, a known heterozygous c.100dupT frameshift (p.Cys34Leufs*4) and the known c.1204G>A missense (p.Gly402Ser) and c.1435C>T nonsense (p.Arg479X) variants as causes of HHT2.
CONCLUSION
Novel and published variants in ENG (37.5%) and ACVRL1 (62.5%) were exclusively identified as the cause of HHT in an Austrian patient cohort. Identification of novel causative genetics variants should facilitate the development of tailored therapeutical applications in the future treatment of autosomal dominant HHT.
MeSH Terms
Figure
Cited by 2 articles
-
Genetic Mutation Analysis Can Supplement Clinically Confirmed Hereditary Hemorrhagic Telangiectasia Populations
Seon Tae Kim
Clin Exp Otorhinolaryngol. 2019;12(4):333-334. doi: 10.21053/ceo.2019.01585.Genetic Variants and Clinical Phenotypes in Korean Patients With Hereditary Hemorrhagic Telangiectasia
Bo-Gyeong Kim, Joo-hyun Jung, Mi-Jung Kim, Eun-Hye Moon, Jae-Hwan Oh, Jung-Woo Park, Heung-Eog Cha, Ju-Hyun Kim, Yoon-Jae Kim, Jun-Won Chung, Ki-Baik Hahm, Hong-Ryul Jin, Yong-Ju Jang, Sung Wan Kim, Seung-Kyu Chung, Dae-Woo Kim, Young Jae Lee, Seon-Tae Kim
Clin Exp Otorhinolaryngol. 2021;14(4):399-406. doi: 10.21053/ceo.2020.02124.
Reference
-
1. Schulte C, Geisthoff U, Lux A, Kupka S, Zenner HP, Blin N, et al. High frequency of ENG and ALK1/ACVRL1 mutations in German HHT patients. Hum Mutat. 2005; Jun. 25(6):595.
Article2. McDonald J, Pyeritz RE. GeneReviews: hereditary hemorrhagic telangiectasia [Internet]. Seattle, WA: University of Washington;2000. [cited 2019 May 25]. Available from: https://www.ncbi.nlm.nih.gov/ books/NBK1351/.3. Westermann CJ, Rosina AF, De Vries V, de Coteau PA. The prevalence and manifestations of hereditary hemorrhagic telangiectasia in the Afro-Caribbean population of the Netherlands Antilles: a family screening. Am J Med Genet A. 2003; Feb. 116(4):324–8.
Article4. Lee ST, Kim JA, Jang SY, Kim DK, Do YS, Suh GY, et al. Clinical features and mutations in the ENG, ACVRL1, and SMAD4 genes in Korean patients with hereditary hemorrhagic telangiectasia. J Korean Med Sci. 2009; Feb. 24(1):69–76.
Article5. Shovlin CL, Guttmacher AE, Buscarini E, Faughnan ME, Hyland RH, Westermann CJ, et al. Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber syndrome). Am J Med Genet. 2000; Mar. 91(1):66–7.
Article6. Abdalla SA, Letarte M. Hereditary haemorrhagic telangiectasia: current views on genetics and mechanisms of disease. J Med Genet. 2006; Feb. 43(2):97–110.
Article7. Begbie ME, Wallace GM, Shovlin CL. Hereditary haemorrhagic telangiectasia (Osler-Weber-Rendu syndrome): a view from the 21st century. Postgrad Med J. 2003; Jan. 79(927):18–24.
Article8. Sadick H, Hage J, Goessler U, Stern-Straeter J, Riedel F, Hoermann K, et al. Mutation analysis of “Endoglin” and “Activin receptor-like kinase” genes in German patients with hereditary hemorrhagic telangiectasia and the value of rapid genotyping using an allele-specific PCR-technique. BMC Med Genet. 2009; Jun. 10:53.
Article9. Letteboer TG, Mager JJ, Snijder RJ, Koeleman BP, Lindhout D, Ploos van Amstel JK, et al. Genotype-phenotype relationship in hereditary haemorrhagic telangiectasia. J Med Genet. 2006; Apr. 43(4):371–7.10. Bayrak-Toydemir P, McDonald J, Akarsu N, Toydemir RM, Calderon F, Tuncali T, et al. A fourth locus for hereditary hemorrhagic telangiectasia maps to chromosome 7. Am J Med Genet A. 2006; Oct. 140(20):2155–62.
Article11. Cole SG, Begbie ME, Wallace GM, Shovlin CL. A new locus for hereditary haemorrhagic telangiectasia (HHT3) maps to chromosome 5. J Med Genet. 2005; Jul. 42(7):577–82.
Article12. Gallione CJ, Repetto GM, Legius E, Rustgi AK, Schelley SL, Tejpar S, et al. A combined syndrome of juvenile polyposis and hereditary haemorrhagic telangiectasia associated with mutations in MADH4 (SMAD4). Lancet. 2004; Mar. 363(9412):852–9.13. Mahmoud M, Upton PD, Arthur HM. Angiogenesis regulation by TGFβ signalling: clues from an inherited vascular disease. Biochem Soc Trans. 2011; Dec. 39(6):1659–66.
Article14. Mahlawat P, Ilangovan U, Biswas T, Sun LZ, Hinck AP. Structure of the Alk1 extracellular domain and characterization of its bone morphogenetic protein (BMP) binding properties. Biochemistry. 2012; Aug. 51(32):6328–41.
Article15. Townson SA, Martinez-Hackert E, Greppi C, Lowden P, Sako D, Liu J, et al. Specificity and structure of a high affinity activin receptor-like kinase 1 (ALK1) signaling complex. J Biol Chem. 2012; Aug. 287(33):27313–25.
Article16. Alt A, Miguel-Romero L, Donderis J, Aristorena M, Blanco FJ, Round A, et al. Structural and functional insights into endoglin ligand recognition and binding. PLoS One. 2012; 7(2):e29948.
Article17. Llorca O, Trujillo A, Blanco FJ, Bernabeu C. Structural model of human endoglin, a transmembrane receptor responsible for hereditary hemorrhagic telangiectasia. J Mol Biol. 2007; Jan. 365(3):694–705.
Article18. Guerrero-Esteo M, Sanchez-Elsner T, Letamendia A, Bernabeu C. Extracellular and cytoplasmic domains of endoglin interact with the transforming growth factor-beta receptors I and II. J Biol Chem. 2002; Aug. 277(32):29197–209.19. Hawinkels LJ, Kuiper P, Wiercinska E, Verspaget HW, Liu Z, Pardali E, et al. Matrix metalloproteinase-14 (MT1-MMP)-mediated endoglin shedding inhibits tumor angiogenesis. Cancer Res. 2010; May. 70(10):4141–50.
Article20. Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006; Jun. 12(6):642–9.
Article21. Letteboer TG, Zewald RA, Kamping EJ, de Haas G, Mager JJ, Snijder RJ, et al. Hereditary hemorrhagic telangiectasia: ENG and ALK-1 mutations in Dutch patients. Hum Genet. 2005; Jan. 116(1-2):8–16.
Article22. Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016; Aug. 536(7616):285–91.23. Lenato GM, Lastella P, Di Giacomo MC, Resta N, Suppressa P, Pasculli G, et al. DHPLC-based mutation analysis of ENG and ALK-1 genes in HHT Italian population. Hum Mutat. 2006; Feb. 27(2):213–4.
Article24. McDonald J, Damjanovich K, Millson A, Wooderchak W, Chibuk JM, Stevenson DA, et al. Molecular diagnosis in hereditary hemorrhagic telangiectasia: findings in a series tested simultaneously by sequencing and deletion/duplication analysis. Clin Genet. 2011; Apr. 79(4):335–44.
Article25. Richards-Yutz J, Grant K, Chao EC, Walther SE, Ganguly A. Update on molecular diagnosis of hereditary hemorrhagic telangiectasia. Hum Genet. 2010; Jul. 128(1):61–77.
Article26. Assis AM, Costa FF, Arruda VR, Annichino-Bizzacchi JM, Bertuzzo CS. Three novel mutations in the activin receptor-like kinase 1 (ALK-1) gene in hereditary hemorrhagic telangiectasia type 2 in Brazilian patients. J Hum Genet. 2007; 52(3):237–43.
Article27. Abdalla SA, Gallione CJ, Barst RJ, Horn EM, Knowles JA, Marchuk DA, et al. Primary pulmonary hypertension in families with hereditary haemorrhagic telangiectasia. Eur Respir J. 2004; Mar. 23(3):373–7.
Article28. Raab U, Velasco B, Lastres P, Letamendia A, Cales C, Langa C, et al. Expression of normal and truncated forms of human endoglin. Biochem J. 1999; May. 339(Pt 3):579–88.
Article29. Berg JN, Gallione CJ, Stenzel TT, Johnson DW, Allen WP, Schwartz CE, et al. The activin receptor-like kinase 1 gene: genomic structure and mutations in hereditary hemorrhagic telangiectasia type 2. Am J Hum Genet. 1997; Jul. 61(1):60–7.
Article30. Olivieri C, Mira E, Delu G, Pagella F, Zambelli A, Malvezzi L, et al. Identification of 13 new mutations in the ACVRL1 gene in a group of 52 unselected Italian patients affected by hereditary haemorrhagic telangiectasia. J Med Genet. 2002; Jul. 39(7):E39.
Article31. Harrison RE, Flanagan JA, Sankelo M, Abdalla SA, Rowell J, Machado RD, et al. Molecular and functional analysis identifies ALK-1 as the predominant cause of pulmonary hypertension related to hereditary haemorrhagic telangiectasia. J Med Genet. 2003; Dec. 40(12):865–71.
Article32. Pawlikowska L, Tran MN, Achrol AS, Ha C, Burchard E, Choudhry S, et al. Polymorphisms in transforming growth factor-beta-related genes ALK1 and ENG are associated with sporadic brain arteriovenous malformations. Stroke. 2005; Oct. 36(10):2278–80.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hereditary Hemorrhagic Telangiectasia in a Family
- Hereditary Hemorrhagic Telangiectasia Combined with Pulmonary Arteriovenous Malformation Treated with Transcatheter Embolotherapy
- A Case of Hereditary Hemorrhagic Telangiectasia
- A Case of Hepatic Involvement in Hereditary Hemorrhagic Telangiectasia Presenting as High Output Heart Failure
- Genetic Mutation Analysis Can Supplement Clinically Confirmed Hereditary Hemorrhagic Telangiectasia Populations