Korean J Physiol Pharmacol.
2015 May;19(3):263-268. 10.4196/kjpp.2015.19.3.263.
LPS Increases 5-LO Expression on Monocytes via an Activation of Akt-Sp1/NF-kappaB Pathways
- Affiliations
-
- 1Department of Pharmacology and BK21 Medical Science Education Center, School of Medicine, Pusan National University, Yangsan 626-870, Korea. chidkim@pusan.ac.kr
- KMID: 1791428
- DOI: http://doi.org/10.4196/kjpp.2015.19.3.263
Abstract
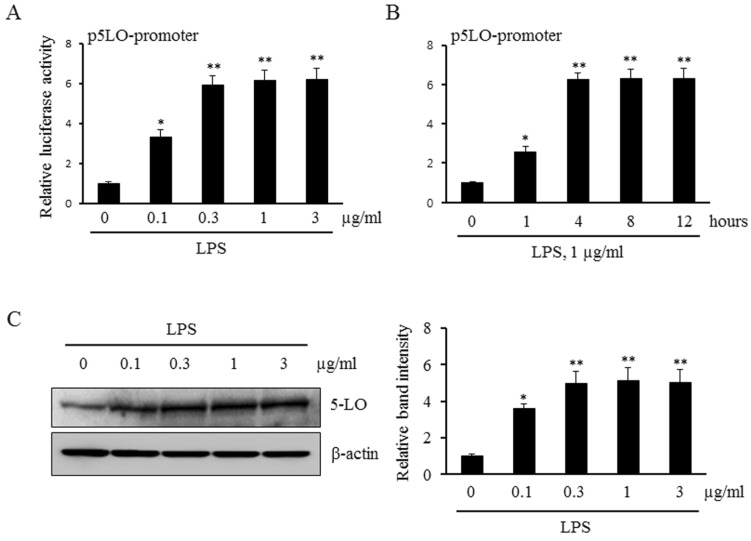
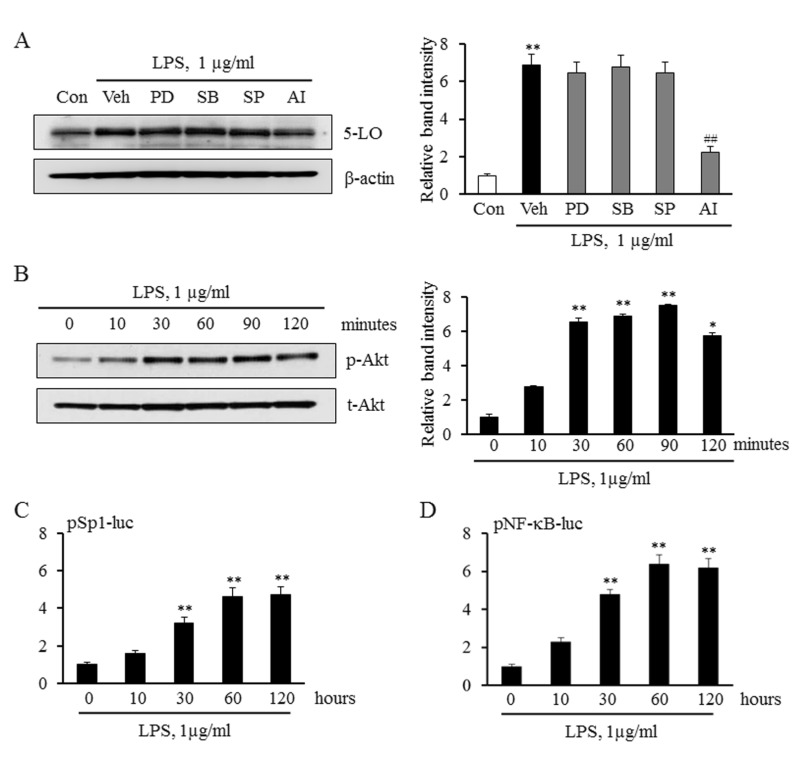
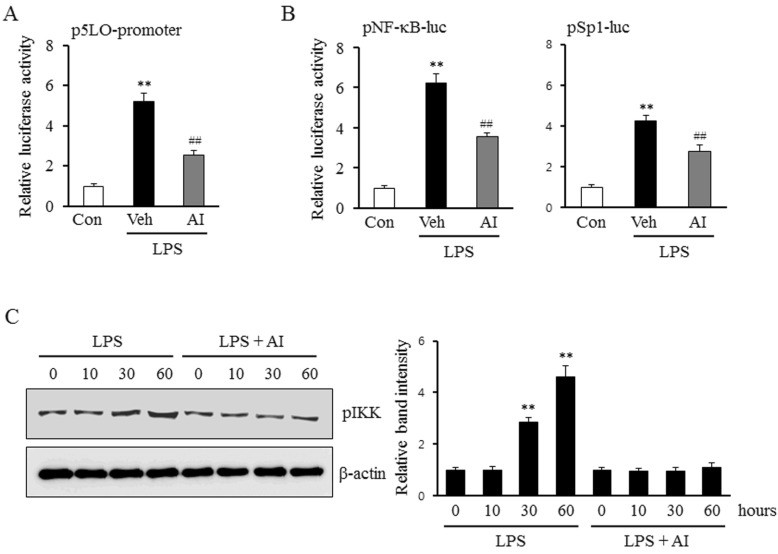
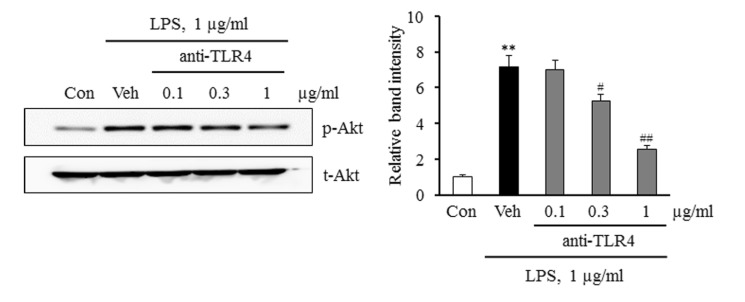
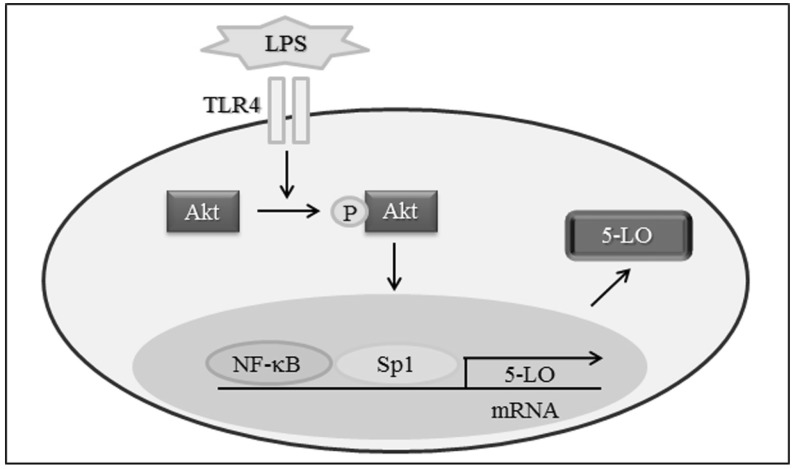
- 5-Lipoxygenase (5-LO) plays a pivotal role in the progression of atherosclerosis. Therefore, this study investigated the molecular mechanisms involved in 5-LO expression on monocytes induced by LPS. Stimulation of THP-1 monocytes with LPS (0~3 microg/ml) increased 5-LO promoter activity and 5-LO protein expression in a concentration-dependent manner. LPS-induced 5-LO expression was blocked by pharmacological inhibition of the Akt pathway, but not by inhibitors of MAPK pathways including the ERK, JNK, and p38 MAPK pathways. In line with these results, LPS increased the phosphorylation of Akt, suggesting a role for the Akt pathway in LPS-induced 5-LO expression. In a promoter activity assay conducted to identify transcription factors, both Sp1 and NF-kappaB were found to play central roles in 5-LO expression in LPS-treated monocytes. The LPS-enhanced activities of Sp1 and NF-kappaB were attenuated by an Akt inhibitor. Moreover, the LPS-enhanced phosphorylation of Akt was significantly attenuated in cells pretreated with an anti-TLR4 antibody. Taken together, 5-LO expression in LPS-stimulated monocytes is regulated at the transcriptional level via TLR4/Akt-mediated activations of Sp1 and NF-kappaB pathways in monocytes.
Keyword
MeSH Terms
Figure
Reference
-
1. Badimon L, Storey RF, Vilahur G. Update on lipids, inflammation and atherothrombosis. Thromb Haemost. 2011; 105(Suppl 1):S34–S42. PMID: 21479344.
Article2. Hansson GK. Inflammatory mechanisms in atherosclerosis. J Thromb Haemost. 2009; 7(Suppl 1):328–331. PMID: 19630827.
Article3. Gupta H, Dai L, Datta G, Garber DW, Grenett H, Li Y, Mishra V, Palgunachari MN, Handattu S, Gianturco SH, Bradley WA, Anantharamaiah GM, White CR. Inhibition of lipopolysaccharide-induced inflammatory responses by an apolipoprotein AI mimetic peptide. Circ Res. 2005; 97:236–243. PMID: 16002747.
Article4. Kuhn AM, Tzieply N, Schmidt MV, von Knethen A, Namgaladze D, Yamamoto M, Brüne B. Antioxidant signaling via Nrf2 counteracts lipopolysaccharide-mediated inflammatory responses in foam cell macrophages. Free Radic Biol Med. 2011; 50:1382–1391. PMID: 21382476.
Article5. Sikorski K, Chmielewski S, Przybyl L, Heemann U, Wesoly J, Baumann M, Bluyssen HA. STAT1-mediated signal integration between IFNγ and LPS leads to increased EC and SMC activation and monocyte adhesion. Am J Physiol Cell Physiol. 2011; 300:C1337–C1344. PMID: 21346151.
Article6. Poeckel D, Funk CD. The 5-lipoxygenase/leukotriene pathway in preclinical models of cardiovascular disease. Cardiovasc Res. 2010; 86:243–253. PMID: 20093252.
Article7. Vila L. Cyclooxygenase and 5-lipoxygenase pathways in the vessel wall: role in atherosclerosis. Med Res Rev. 2004; 24:399–424. PMID: 15170590.
Article8. Mehrabian M, Allayee H. 5-lipoxygenase and atherosclerosis. Curr Opin Lipidol. 2003; 14:447–457. PMID: 14501583.
Article9. Yonekawa K, Neidhart M, Altwegg LA, Wyss CA, Corti R, Vogl T, Grigorian M, Gay S, Lüscher TF, Maier W. Myeloid related proteins activate Toll-like receptor 4 in human acute coronary syndromes. Atherosclerosis. 2011; 218:486–492. PMID: 21782178.
Article10. Kawamoto T, Ii M, Kitazaki T, Iizawa Y, Kimura H. TAK-242 selectively suppresses Toll-like receptor 4-signaling mediated by the intracellular domain. Eur J Pharmacol. 2008; 584:40–48. PMID: 18299127.
Article11. Serio KJ, Reddy KV, Bigby TD. Lipopolysaccharide induces 5-lipoxygenase-activating protein gene expression in THP-1 cells via a NF-kappaB and C/EBP-mediated mechanism. Am J Physiol Cell Physiol. 2005; 288:C1125–C1133. PMID: 15625306.12. Zhao L, Moos MP, Gräbner R, Pédrono F, Fan J, Kaiser B, John N, Schmidt S, Spanbroek R, Lötzer K, Huang L, Cui J, Rader DJ, Evans JF, Habenicht AJ, Funk CD. The 5-lipoxygenase pathway promotes pathogenesis of hyperlipidemia-dependent aortic aneurysm. Nat Med. 2004; 10:966–973. PMID: 15322539.
Article13. De Caterina R, Zampolli A. From asthma to atherosclerosis--5-lipoxygenase, leukotrienes, and inflammation. N Engl J Med. 2004; 350:4–7. PMID: 14702420.14. Jawień J. The putative role of leukotrienes in experimental atherogenesis. Pol Arch Med Wewn. 2009; 119:90–93. PMID: 19341185.
Article15. Rådmark O, Werz O, Steinhilber D, Samuelsson B. 5-Lipoxygenase: regulation of expression and enzyme activity. Trends Biochem Sci. 2007; 32:332–341. PMID: 17576065.
Article16. Lötzer K, Funk CD, Habenicht AJ. The 5-lipoxygenase pathway in arterial wall biology and atherosclerosis. Biochim Biophys Acta. 2005; 1736:30–37. PMID: 16081317.17. Yang HJ, Youn H, Seong KM, Yun YJ, Kim W, Kim YH, Lee JY, Kim CS, Jin YW, Youn B. Psoralidin, a dual inhibitor of COX-2 and 5-LOX, regulates ionizing radiation (IR)-induced pulmonary inflammation. Biochem Pharmacol. 2011; 82:524–534. PMID: 21669192.
Article18. Sánchez-Galán E, Gómez-Hernández A, Vidal C, Martín-Ventura JL, Blanco-Colio LM, Muñoz-García B, Ortega L, Egido J, Tuñón J. Leukotriene B4 enhances the activity of nuclear factorkappaB pathway through BLT1 and BLT2 receptors in atherosclerosis. Cardiovasc Res. 2009; 81:216–225. PMID: 18852255.19. Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011; 12:204–212. PMID: 21321594.
Article20. Drüeke TB, Massy ZA. Atherosclerosis in CKD: differences from the general population. Nat Rev Nephrol. 2010; 6:723–735. PMID: 20978469.
Article21. Gitlin JM, Loftin CD. Cyclooxygenase-2 inhibition increases lipopolysaccharide-induced atherosclerosis in mice. Cardiovasc Res. 2009; 81:400–407. PMID: 18948273.
Article22. Szeto CC, Kwan BC, Chow KM, Lai KB, Chung KY, Leung CB, Li PK. Endotoxemia is related to systemic inflammation and atherosclerosis in peritoneal dialysis patients. Clin J Am Soc Nephrol. 2008; 3:431–436. PMID: 18256376.
Article23. Lalla E, Lamster IB, Hofmann MA, Bucciarelli L, Jerud AP, Tucker S, Lu Y, Papapanou PN, Schmidt AM. Oral infection with a periodontal pathogen accelerates early atherosclerosis in apolipoprotein E-null mice. Arterioscler Thromb Vasc Biol. 2003; 23:1405–1411. PMID: 12816879.
Article24. Serio KJ, Reddy KV, Bigby TD. Lipopolysaccharide induces 5-lipoxygenase-activating protein gene expression in THP-1 cells via a NF-kappaB and C/EBP-mediated mechanism. Am J Physiol Cell Physiol. 2005; 288:C1125–C1133. PMID: 15625306.25. Lee SJ, Kim CE, Seo KW, Kim CD. HNE-induced 5-LO expression is regulated by NF-{kappa}B/ERK and Sp1/p38 MAPK pathways via EGF receptor in murine macrophages. Cardiovasc Res. 2010; 88:352–359. PMID: 20554538.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- CKD-712, (S)-1-(alpha-naphthylmethyl)-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline, Inhibits the NF-kappaB Activation and Augments Akt Activation during TLR4 Signaling
- NF-kappaB Activation in T Helper 17 Cell Differentiation
- Silymarin Inhibits Morphological Changes in LPS-Stimulated Macrophages by Blocking NF-kappaB Pathway
- Prostaglandin E2 Induces IL-6 and IL-8 Production by the EP Receptors/Akt/NF-kappaB Pathways in Nasal Polyp-Derived Fibroblasts
- The Regulatory Mechanism of Src Familly Kinase in Lipopolysaccharide (LPS) induced HF-kappaB Activation Pathway