Korean J Physiol Pharmacol.
2015 May;19(3):203-210. 10.4196/kjpp.2015.19.3.203.
EGCG Blocked Phenylephrin-Induced Hypertrophy in H9C2 Cardiomyocytes, by Activating AMPK-Dependent Pathway
- Affiliations
-
- 1Guangzhou Research Institute of Snake Venom, China. yicaisysu@163.com
- 2Department of Pharmacology, Guangzhou Medical University, Guangzhou, 510182, Guangdong, P.R. China. wuxiaoqian2004@hotmail.com
- KMID: 1791420
- DOI: http://doi.org/10.4196/kjpp.2015.19.3.203
Abstract
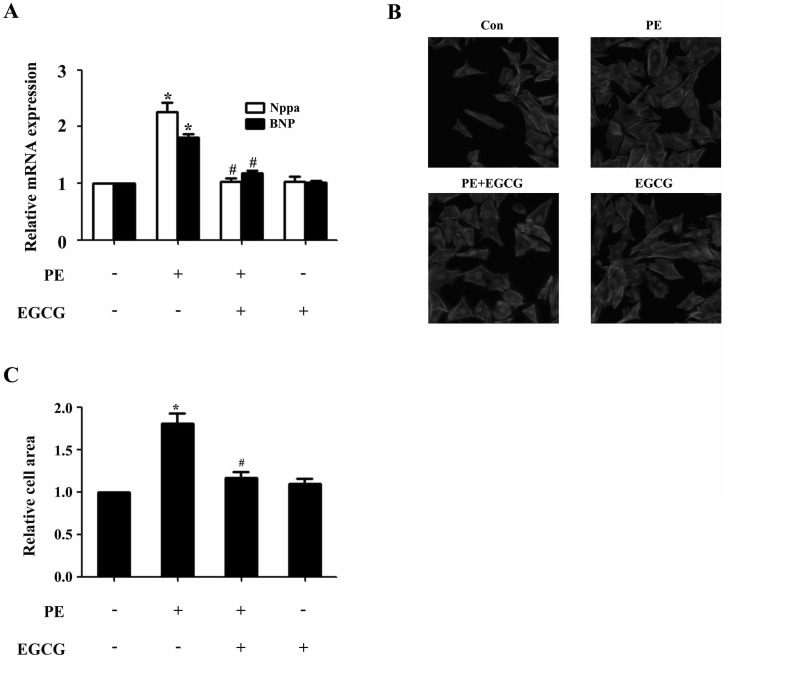
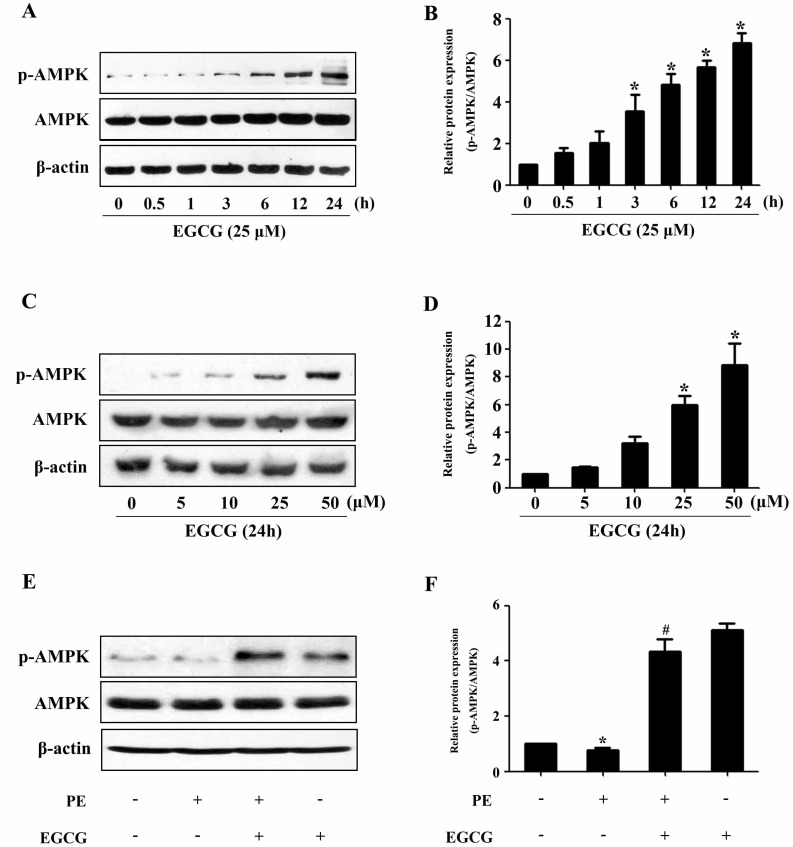
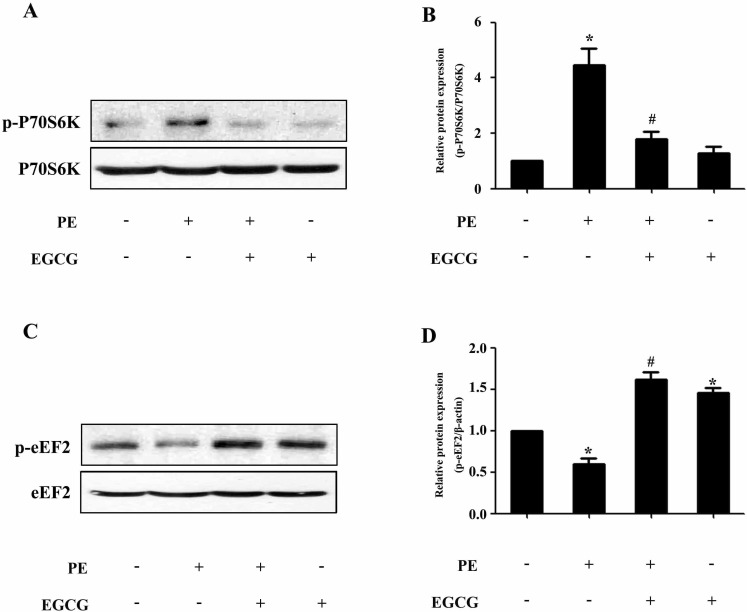
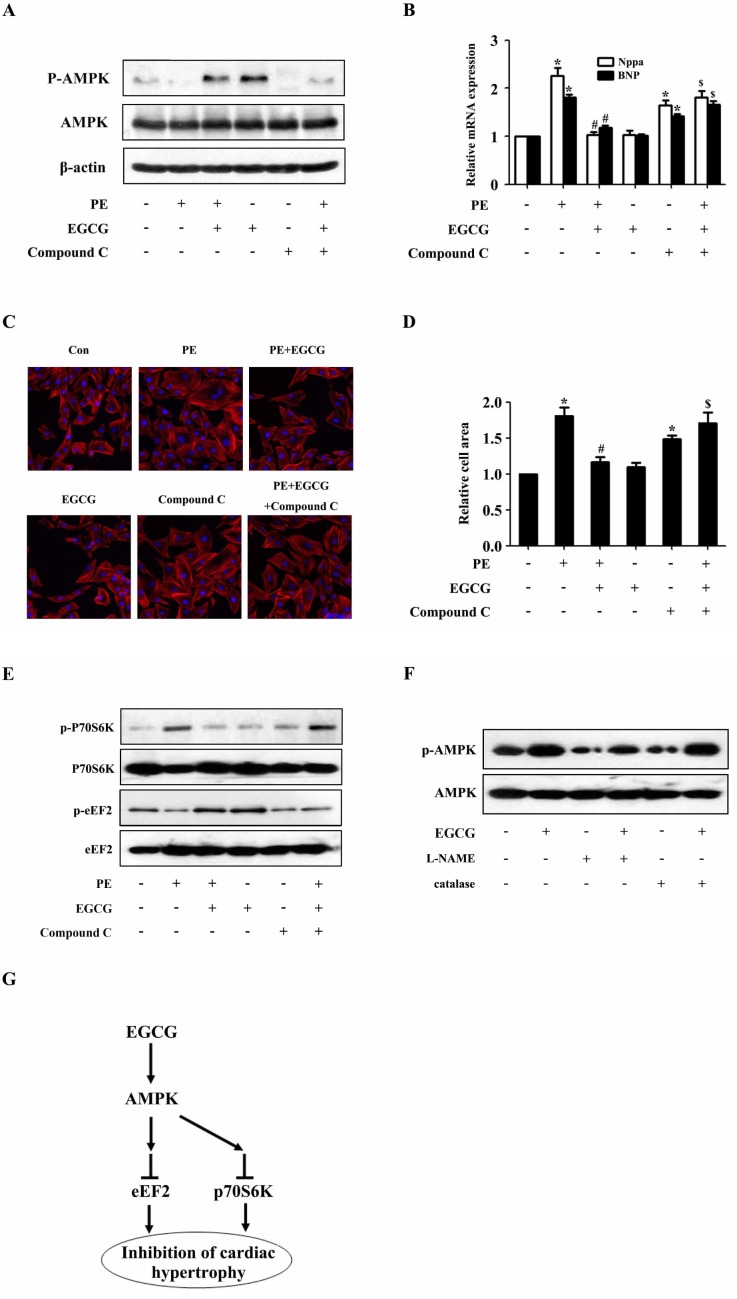
- AMP-activated protein kinase (AMPK) is a key regulator of energy metabolism. Previous studies have shown that activation of AMPK results in suppression of cardiac myocyte hypertrophy via inhibition of the p70S6 kinase (p70S6K) and eukaryotic elongation factor-2 (eEF2) signaling pathways. Epigallocatechin-3-gallate (EGCG), the major polyphenol found in green tea, possesses multiple protective effects on the cardiovascular system including cardiac hypertrophy. However, the molecular mechanisms has not been well investigated. In this study, we found that EGCG could significantly reduce natriuretic peptides type A (Nppa), brain natriuretic polypeptide (BNP) mRNA expression and decrease cell surface area in H9C2 cardiomyocytes stimulated with phenylephrine (PE). Moreover, we showed that AMPK is activated in H9C2 cardiomyocytes by EGCG, and AMPK-dependent pathway participates in the inhibitory effects of EGCG on cardiac hypertrophy. Taken together, our findings provide the first evidence that the effect of EGCG against cardiac hypertrophy may be attributed to its activation on AMPK-dependent signaling pathway, suggesting the therapeutic potential of EGCG on the prevention of cardiac remodeling in patients with pressure overload hypertrophy.
Keyword
MeSH Terms
Figure
Reference
-
1. Pillai VB, Sundaresan NR, Gupta MP. Regulation of Akt signaling by sirtuins: its implication in cardiac hypertrophy and aging. Circ Res. 2014; 114:368–378. PMID: 24436432.2. Oka T, Akazawa H, Naito AT, Komuro I. Angiogenesis and cardiac hypertrophy: maintenance of cardiac function and causative roles in heart failure. Circ Res. 2014; 114:565–571. PMID: 24481846.3. Cha HN, Choi JH, Kim YW, Kim JY, Ahn MW, Park SY. Metformin inhibits isoproterenol-induced cardiac hypertrophy in mice. Korean J Physiol Pharmacol. 2010; 14:377–384. PMID: 21311678.
Article4. Mudd JO, Kass DA. Tackling heart failure in the twenty-first century. Nature. 2008; 451:919–928. PMID: 18288181.
Article5. Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB;. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014; 129:e28–292. PMID: 24352519.6. Messerli FH, Bangalore S, Yao SS, Steinberg JS. Cardioprotection with beta-blockers: myths, facts and Pascal's wager. J Intern Med. 2009; 266:232–241. PMID: 19702791.
Article7. Nolin TD, Himmelfarb J. Mechanisms of drug-induced nephrotoxicity. Handb Exp Pharmacol. 2010; (196):111–130. PMID: 20020261.
Article8. Wolfram S. Effects of green tea and EGCG on cardiovascular and metabolic health. J Am Coll Nutr. 2007; 26:373S–3388. PMID: 17906191.
Article9. Cai Y, Yu SS, Chen TT, Gao S, Geng B, Yu Y, Ye JT, Liu PQ. EGCG inhibits CTGF expression via blocking NF-κB activation in cardiac fibroblast. Phytomedicine. 2013; 20:106–113. PMID: 23141425.
Article10. Li HL, Huang Y, Zhang CN, Liu G, Wei YS, Wang AB, Liu YQ, Hui RT, Wei C, Williams GM, Liu DP, Liang CC. Epigallocathechin-3 gallate inhibits cardiac hypertrophy through blocking reactive oxidative species-dependent and -independent signal pathways. Free Radic Biol Med. 2006; 40:1756–1775. PMID: 16767845.
Article11. Beauloye C, Bertrand L, Horman S, Hue L. AMsPK activation, a preventive therapeutic target in the transition from cardiac injury to heart failure. Cardiovasc Res. 2011; 90:224–233. PMID: 21285292.12. Kim SJ, Lee JH, Chung HS, Song JH, Ha J, Bae H. Neuroprotective effects of amp-activated protein kinase on scopolamine induced memory impairment. Korean J Physiol Pharmacol. 2013; 17:331–338. PMID: 23946693.
Article13. Chan AY, Soltys CL, Young ME, Proud CG, Dyck JR. Activation of AMP-activated protein kinase inhibits protein synthesis associated with hypertrophy in the cardiac myocyte. J Biol Chem. 2004; 279:32771–32779. PMID: 15159410.
Article14. Shioi T, McMullen JR, Tarnavski O, Converso K, Sherwood MC, Manning WJ, Izumo S. Rapamycin attenuates load-induced cardiac hypertrophy in mice. Circulation. 2003; 107:1664–1670. PMID: 12668503.
Article15. Zarrinpashneh E, Beauloye C, Ginion A, Pouleur AC, Havaux X, Hue L, Viollet B, Vanoverschelde JL, Bertrand L. AMPKalpha2 counteracts the development of cardiac hypertrophy induced by isoproterenol. Biochem Biophys Res Commun. 2008; 376:677–681. PMID: 18812163.16. Zhang P, Hu X, Xu X, Fassett J, Zhu G, Viollet B, Xu W, Wiczer B, Bernlohr DA, Bache RJ, Chen Y. AMP activated protein kinase-alpha2 deficiency exacerbates pressure-overload-induced left ventricular hypertrophy and dysfunction in mice. Hypertension. 2008; 52:918–924. PMID: 18838626.17. Murase T, Misawa K, Haramizu S, Hase T. Catechin-induced activation of the LKB1/AMP-activated protein kinase pathway. Biochem Pharmacol. 2009; 78:78–84. PMID: 19447226.
Article18. Zhou J, Farah BL, Sinha RA, Wu Y, Singh BK, Bay BH, Yang CS, Yen PM. Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, stimulates hepatic autophagy and lipid clearance. PLoS One. 2014; 9:e87161. PMID: 24489859.
Article19. Kim S, Jung J, Kim H, Heo RW, Yi CO, Lee JE, Jeon BT, Kim WH, Hahm JR, Roh GS. Exendin-4 Improves Nonalcoholic Fatty Liver Disease by Regulating Glucose Transporter 4 Expression in ob/ob Mice. Korean J Physiol Pharmacol. 2014; 18:333–339. PMID: 25177166.
Article20. Zhong Y, Chiou YS, Pan MH, Shahidi F. Anti-inflammatory activity of lipophilic epigallocatechin gallate (EGCG) derivatives in LPS-stimulated murine macrophages. Food Chem. 2012; 134:742–748. PMID: 23107686.
Article21. Valenti D, de Bari L, Manente GA, Rossi L, Mutti L, Moro L, Vacca RA. Negative modulation of mitochondrial oxidative phosphorylation by epigallocatechin-3 gallate leads to growth arrest and apoptosis in human malignant pleural mesothelioma cells. Biochim Biophys Acta. 2013; 1832:2085–2096. PMID: 23911347.
Article22. Cai Y, Kurita-Ochiai T, Hashizume T, Yamamoto M. Green tea epigallocatechin-3-gallate attenuates Porphyromonas gingivalis-induced atherosclerosis. Pathog Dis. 2013; 67:76–83. PMID: 23620122.23. Priyadarshi S, Valentine B, Han C, Fedorova OV, Bagrov AY, Liu J, Periyasamy SM, Kennedy D, Malhotra D, Xie Z, Shapiro JI. Effect of green tea extract on cardiac hypertrophy following 5/6 nephrectomy in the rat. Kidney Int. 2003; 63:1785–1790. PMID: 12675854.
Article24. Hardie DG, Ashford ML. AMPK: regulating energy balance at the cellular and whole body levels. Physiology (Bethesda). 2014; 29:99–107. PMID: 24583766.
Article25. Park SY, Kim MH, Ahn JH, Lee SJ, Lee JH, Eum WS, Choi SY, Kwon HY. The stimulatory effect of essential fatty acids on glucose uptake involves both Akt and AMPK activation in C2C12 skeletal muscle cells. Korean J Physiol Pharmacol. 2014; 18:255–261. PMID: 24976766.
Article26. Hernández JS, Barreto-Torres G, Kuznetsov AV, Khuchua Z, Javadov S. Crosstalk between AMPK activation and angiotensin II-induced hypertrophy in cardiomyocytes: the role of mitochondria. J Cell Mol Med. 2014; 18:709–720. PMID: 24444314.27. Zhuo L, Fu B, Bai X, Zhang B, Wu L, Cui J, Cui S, Wei R, Chen X, Cai G. NAD blocks high glucose induced mesangial hypertrophy via activation of the sirtuins-AMPK-mTOR pathway. Cell Physiol Biochem. 2011; 27:681–690. PMID: 21691086.
Article28. Kang S, Chemaly ER, Hajjar RJ, Lebeche D. Resistin promotes cardiac hypertrophy via the AMP-activated protein kinase/mammalian target of rapamycin (AMPK/mTOR) and c-Jun N-terminal kinase/insulin receptor substrate 1 (JNK/IRS1) pathways. J Biol Chem. 2011; 286:18465–18473. PMID: 21478152.
Article29. Chan AY, Dolinsky VW, Soltys CL, Viollet B, Baksh S, Light PE, Dyck JR. Resveratrol inhibits cardiac hypertrophy via AMP-activated protein kinase and Akt. J Biol Chem. 2008; 283:24194–24201. PMID: 18562309.
Article30. Qiao W, Zhang W, Gai Y, Zhao L, Fan J. The histone acetyltransferase MOF overexpression blunts cardiac hypertrophy by targeting ROS in mice. Biochem Biophys Res Commun. 2014; 448:379–384. PMID: 24802406.
Article31. Sandström ME, Zhang SJ, Bruton J, Silva JP, Reid MB, Westerblad H, Katz A. Role of reactive oxygen species in contraction-mediated glucose transport in mouse skeletal muscle. J Physiol. 2006; 575:251–262. PMID: 16777943.
Article32. Hwang JT, Ha J, Park IJ, Lee SK, Baik HW, Kim YM, Park OJ. Apoptotic effect of EGCG in HT-29 colon cancer cells via AMPK signal pathway. Cancer Lett. 2007; 247:115–121. PMID: 16797120.
Article33. Choi HC, Song P, Xie Z, Wu Y, Xu J, Zhang M, Dong Y, Wang S, Lau K, Zou MH. Reactive nitrogen species is required for the activation of the AMP-activated protein kinase by statin in vivo. J Biol Chem. 2008; 283:20186–20197. PMID: 18474592.
Article34. Zou MH, Kirkpatrick SS, Davis BJ, Nelson JS, Wiles WG 4th, Schlattner U, Neumann D, Brownlee M, Freeman MB, Goldman MH. Activation of the AMP-activated protein kinase by the anti-diabetic drug metformin in vivo. Role of mitochondrial reactive nitrogen species. J Biol Chem. 2004; 279:43940–43951. PMID: 15265871.35. Thandapilly SJ, Louis XL, Yang T, Stringer DM, Yu L, Zhang S, Wigle J, Kardami E, Zahradka P, Taylor C, Anderson HD, Netticadan T. Resveratrol prevents norepinephrine induced hypertrophy in adult rat cardiomyocytes, by activating NO-AMPK pathway. Eur J Pharmacol. 2011; 668:217–224. PMID: 21756902.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Benzoylaconine improves mitochondrial function in oxygenglucose deprivation and reperfusion-induced cardiomyocyte injury by activation of the AMPK/PGC-1 axis
- Adriamycin Induced Apoptosis of H9c2 Cardiomyocytes via a Caspase-Independent Pathway
- Inhibition of Amyloid beta Peptide-induced Neuronal Cytotoxicity by EGCG
- Acetaminophen Induced Cytotoxicity and Altered Gene Expression in Cultured Cardiomyocytes of H9C2 Cells
- Nicorandil alleviated cardiac hypoxia/reoxygenation-induced cytotoxicity via upregulating ketone body metabolism and ACAT1 activity