Korean J Physiol Pharmacol.
2019 Jan;23(1):37-45. 10.4196/kjpp.2019.23.1.37.
Nicorandil alleviated cardiac hypoxia/reoxygenation-induced cytotoxicity via upregulating ketone body metabolism and ACAT1 activity
- Affiliations
-
- 1Department of Cardiology, The Affiliated Hospital of Yan'an University, Yan'an 716000, China.
- 2Department of Cardiology, The Forth Renmin Hospital of Xi'an, Shanxi Province 710004, China. hanleisen196601@126.com
- KMID: 2443614
- DOI: http://doi.org/10.4196/kjpp.2019.23.1.37
Abstract
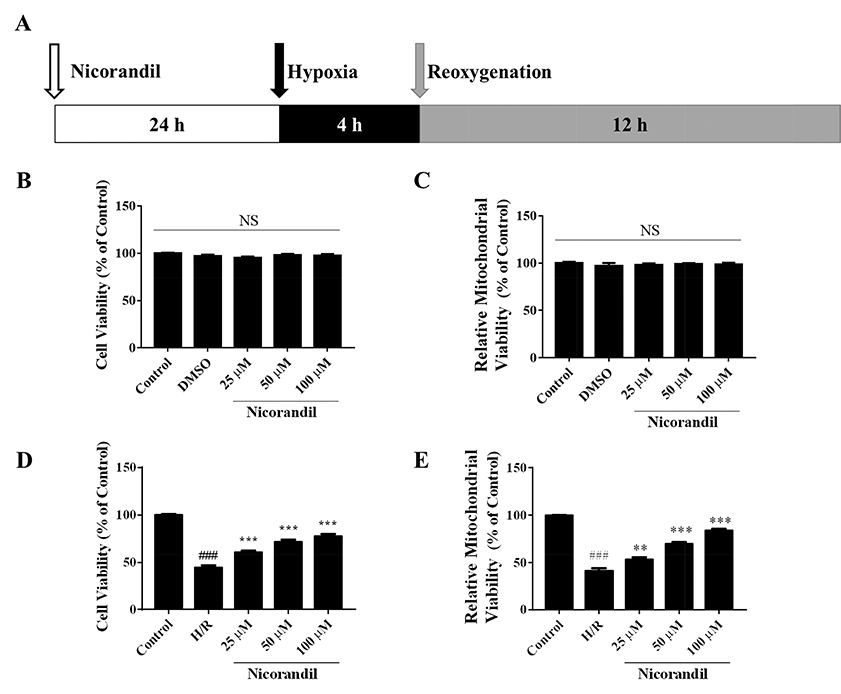
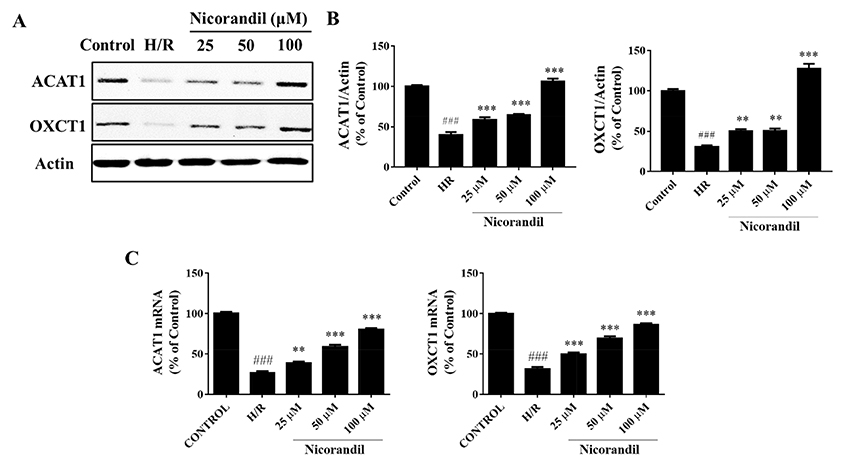
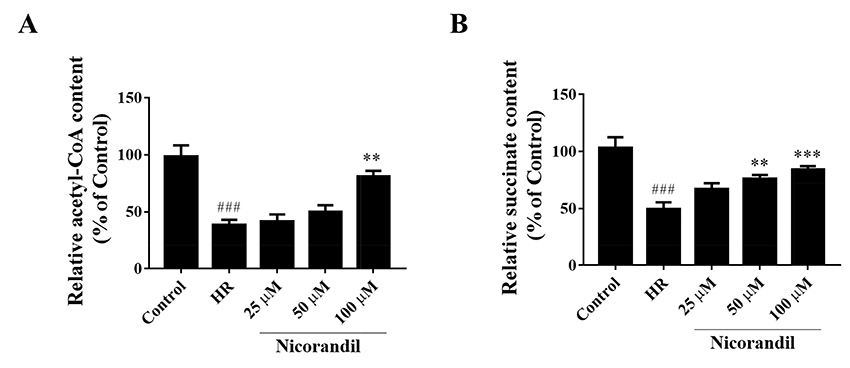
- To study the effect of nicorandil pretreatment on ketone body metabolism and Acetyl-CoA acetyltransferase (ACAT1) activity in hypoxia/reoxygenation (H/R)-induced cardiomyocytes. In our study, we applied H9c2 cardiomyocytes cell line to evaluate the cardioprotective effects of nicorandil. We detected mitochondrial viability, cellular apoptosis, reactive oxygen species (ROS) production and calcium overloading in H9c2 cells that exposed to H/R-induced cytotoxicity. Then we evaluated whether nicorandil possibly regulated ketone body, mainly β-hydroxybutyrate (BHB) and acetoacetate (ACAC), metabolism by regulating ACAT1 and Succinyl-CoA:3-keto-acid coenzyme A transferase 1 (OXCT1) protein and gene expressions. Nicorandil protected H9c2 cardiomyocytes against H/R-induced cytotoxicity dose-dependently by mitochondria-mediated anti-apoptosis pathway. Nicorandil significantly decreased cellular apoptotic rate and enhanced the ratio of Bcl-2/Bax expressions. Further, nicorandil decreased the production of ROS and alleviated calcium overloading in H/R-induced H9c2 cells. In crucial, nicorandil upregulated ACAT1 and OXCT1 protein expressions and either of their gene expressions, contributing to increased production of cellular BHB and ACAC. Nicorandil alleviated cardiomyocytes H/R-induced cytotoxicity through upregulating ACAT1/OXCT1 activity and ketone body metabolism, which might be a potential mechanism for emerging study of nicorandil and other K(ATP) channel openers.
MeSH Terms
Figure
Reference
-
1. Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007; 357:1121–1135.
Article2. Silachev DN, Plotnikov EY, Pevzner IB, Zorova LD, Babenko VA, Zorov SD, Popkov VA, Jankauskas SS, Zinchenko VP, Sukhikh GT, Zorov DB. The mitochondrion as a key regulator of ischaemic tolerance and injury. Heart Lung Circ. 2014; 23:897–904.
Article3. Abete P, Testa G, Cacciatore F, Della-Morte D, Galizia G, Langellotto A, Rengo F. Ischemic preconditioning in the younger and aged heart. Aging Dis. 2011; 2:138–148.4. Kloner RA, Rezkalla SH. Preconditioning, postconditioning and their application to clinical cardiology. Cardiovasc Res. 2006; 70:297–307.
Article5. Fukao T, Mitchell G, Sass JO, Hori T, Orii K, Aoyama Y. Ketone body metabolism and its defects. J Inherit Metab Dis. 2014; 37:541–551.
Article6. Cotter DG, Schugar RC, Crawford PA. Ketone body metabolism and cardiovascular disease. Am J Physiol Heart Circ Physiol. 2013; 304:H1060–H1076.
Article7. Faria MH, Muniz LR, Vasconcelos PR. Ketone bodies metabolism during ischemic and reperfusion brain injuries following bilateral occlusion of common carotid arteries in rats. Acta Cir Bras. 2007; 22:125–129.
Article8. Tieu K, Perier C, Caspersen C, Teismann P, Wu DC, Yan SD, Naini A, Vila M, Jackson-Lewis V, Ramasamy R, Przedborski S. D-β-hydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson disease. J Clin Invest. 2003; 112:892–901.
Article9. Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, Le Moan N, Grueter CA, Lim H, Saunders LR, Stevens RD, Newgard CB, Farese RV Jr, de Cabo R, Ulrich S, Akassoglou K, Verdin E. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 2013; 339:211–214.
Article10. Nagao M, Toh R, Irino Y, Mori T, Nakajima H, Hara T, Honjo T, Satomi-Kobayashi S, Shinke T, Tanaka H, Ishida T, Hirata K. β-Hydroxybutyrate elevation as a compensatory response against oxidative stress in cardiomyocytes. Biochem Biophys Res Commun. 2016; 475:322–328.
Article11. Thaler S, Choragiewicz TJ, Rejdak R, Fiedorowicz M, Turski WA, Tulidowicz-Bielak M, Zrenner E, Schuettauf F, Zarnowski T. Neuroprotection by acetoacetate and β-hydroxybutyrate against NMDA-induced RGC damage in rat--possible involvement of kynurenic acid. Graefes Arch Clin Exp Ophthalmol. 2010; 248:1729–1735.
Article12. Massieu L, Haces ML, Montiel T, Hernández-Fonseca K. Acetoacetate protects hippocampal neurons against glutamate-mediated neuronal damage during glycolysis inhibition. Neuroscience. 2003; 120:365–378.
Article13. Schugar RC, Moll AR, André d'Avignon D, Weinheimer CJ, Kovacs A, Crawford PA. Cardiomyocyte-specific deficiency of ketone body metabolism promotes accelerated pathological remodeling. Mol Metab. 2014; 3:754–769.
Article14. Li W, Wu N, Shu W, Jia D, Jia P. Pharmacological preconditioning and postconditioning with nicorandil attenuates ischemia/reperfusion-induced myocardial necrosis and apoptosis in hypercholesterolemic rats. Exp Ther Med. 2015; 10:2197–2205.
Article15. Ishii H, Ichimiya S, Kanashiro M, Amano T, Imai K, Murohara T, Matsubara T. Impact of a single intravenous administration of nicorandil before reperfusion in patients with ST-segment-elevation myocardial infarction. Circulation. 2005; 112:1284–1288.
Article16. Wu H, Ye M, Yang J, Ding J, Yang J, Dong W, Wang X. Nicorandil protects the heart from ischemia/reperfusion injury by attenuating endoplasmic reticulum response-induced apoptosis through PI3K/Akt signaling pathway. Cell Physiol Biochem. 2015; 35:2320–2332.
Article17. Dhalla NS, Elmoselhi AB, Hata T, Makino N. Status of myocardial antioxidants in ischemia-reperfusion injury. Cardiovasc Res. 2000; 47:446–456.
Article18. Newman JC, Verdin E. Ketone bodies as signaling metabolites. Trends Endocrinol Metab. 2014; 25:42–52.
Article19. Aubert G, Martin OJ, Horton JL, Lai L, Vega RB, Leone TC, Koves T, Gardell SJ, Krüger M, Hoppel CL, Lewandowski ED, Crawford PA, Muoio DM, Kelly DP. The failing heart relies on ketone bodies as a fuel. Circulation. 2016; 133:698–705.
Article20. Bedi KC Jr, Snyder NW, Brandimarto J, Aziz M, Mesaros C, Worth AJ, Wang LL, Javaheri A, Blair IA, Margulies KB, Rame JE. Evidence for intramyocardial disruption of lipid metabolism and increased myocardial ketone utilization in advanced human heart failure. Circulation. 2016; 133:706–716.
Article21. Crawford PA, Crowley JR, Sambandam N, Muegge BD, Costello EK, Hamady M, Knight R, Gordon JI. Regulation of myocardial ketone body metabolism by the gut microbiota during nutrient deprivation. Proc Natl Acad Sci U S A. 2009; 106:11276–11281.
Article22. Camberos-Luna L, Gerónimo-Olvera C, Montiel T, Rincon-Heredia R, Massieu L. The ketone body, β-hydroxybutyrate stimulates the autophagic flux and prevents neuronal death induced by glucose deprivation in cortical cultured neurons. Neurochem Res. 2016; 41:600–609.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Corrigendum to: Nicorandil alleviated cardiac hypoxia/reoxygenation-induced cytotoxicity via upregulating ketone body metabolism and ACAT1 activity
- Effect of Propofol on Kupffer Cell Superoxide Dismutase Activities and Cytoprotections during Hypoxia-Reoxygenation
- EFFECTS OF OXYGEN FREE RADICAL SCAVENGERS ON THE HYPOXIA-REOXYGENATION INDUCED PROLIFERATION OF CULTURED HUMAN FIBROBLAST MALME-3 CELL LINE
- Reoxygenation stimulates EDRF(s) release from endothelial cells of rabbit aorta
- Effects of Hypoxia/Reoxygenation and Sodium Nitroprusside on Acid Secretion and Cyclic Nucleotide of Isolated Gastric Cells