J Korean Med Sci.
2014 Feb;29(2):265-271. 10.3346/jkms.2014.29.2.265.
Microarray Analysis for Genes Associated with Angiogenesis in Diabetic OLETF Keratocytes
- Affiliations
-
- 1Department of Ophthalmology, Busan St. Mary's Hospital, Busan, Korea.
- 2Department of Ophthalmology, School of Medicine, Pusan National University and Medical Research Institute, Pusan National University Hospital, Busan, Korea. jongsool@pusan.ac.kr
- KMID: 1789986
- DOI: http://doi.org/10.3346/jkms.2014.29.2.265
Abstract
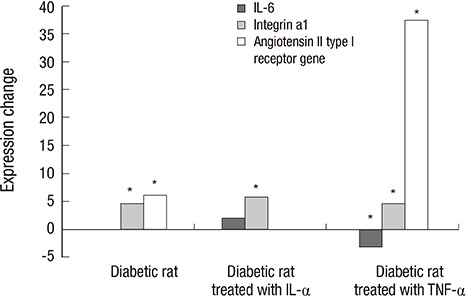
- The purpose of this study was to identify the differences in angiogenesis gene expression between normal and diabetic keratocytes stimulated with interleukin-1alpha (IL-1alpha) and tumor necrosis factor-alpha (TNF-alpha). Primarily cultured normal and diabetic keratocytes were treated with 20 ng/mL of IL-1a and TNF-alpha for 6 hr. cDNA was hybridized to an oligonucleotide microarray. Microarray analysis was used to identify differentially expressed genes that were further evaluated by real-time polymerase chain reaction (RT-PCR). Diabetes keratocytes overexpressed vital components of angiogenesis including Agtr1, and under-expressed components related to the blood vessel maturation, including Dcn. Cytokine-treated diabetic keratocytes differentially expressed components of angiogenesis. OLETF keratocytes after treatment with IL-1alpha and TNF-alpha showed the newly expressed 15 and 14 genes, respectively. Newly and commonly under-expressed five genes followed by treatment with both IL-1alpha and TNF-alpha were also evident. RT-PCR showed results similar to the microarray results. Agtr1 and Itga1 showed an increased expression in diabetic keratocytes compared with normal corneal keratocytes, especially after TNF-alpha treatment. Il6 appeared strong expression after interleukin-1alpha treatment, but showed down expression after TNF-alpha treatment. Further studies to analyze and confirm the significance of the identified angiogenetic genes of diabetes are needed.
MeSH Terms
-
Animals
Cells, Cultured
Gene Expression Regulation/drug effects
Interleukin-1alpha/pharmacology
Keratinocytes/cytology/drug effects/*metabolism
Neovascularization, Physiologic/*genetics
*Oligonucleotide Array Sequence Analysis
Rats
Real-Time Polymerase Chain Reaction
Receptor, Angiotensin, Type 1/genetics/metabolism
Tumor Necrosis Factor-alpha/pharmacology
Interleukin-1alpha
Receptor, Angiotensin, Type 1
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Folkman J. Tumor angiogenesis. In : Mendelsohn J, Howley PM, Israel MA, Liotta LA, editors. The molecular basis of cancer. Philadephia: WB Saunders;1995. p. 206–232.2. Ma JJ, Adamis AP. Corneal angiogenesis. In : Foster CS, Azar DT, Dohlman CH, editors. Smolin and Thoft's the cornea scientific foundation and clinical practice. 4th ed. Philadelphia: Williams & Wilkins;2005. p. 141–149.3. Kim SO, Lee HS, Ahn K, Park K. COMP-angiopoietin-1 promotes cavernous angiogenesis in a type 2 diabetic rat model. J Korean Med Sci. 2013; 28:725–730.4. Folkman J, Klagsburn M. Angiogenic factors. Science. 1987; 235:442–447.5. Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol. 1995; 11:73–91.6. Casey R, Li WW. Factors controlling ocular angiogenesis. Am J Ophthalmol. 1997; 124:521–529.7. D'Amore PA. Mechanisms of retinal and choroidal neovascularization. Invest Ophthalmol Vis Sci. 1994; 35:3974–3979.8. Kim JM, Kim JY, Lee YH, Choi GJ. Angiogenesis according to expressive change of angiogenic related factor in human RPE under oxidative stress. J Korean Ophthalmol Soc. 2005; 46:366–376.9. Kenyon BM, Voest EE, Chen CC, Flynn E, Folkman J, D'Amato RJ. A model of angiogenesis in the mouse cornea. Invest Ophthalmol Vis Sci. 1996; 37:1625–1632.10. Lee JB, Jung SE, Lee HC, Kwon HM, Hong BK, Park HY, Kim EK, Oh JH. Effect of vascular endothelial growth factor (VEGF) on the corneal neovascularization and expression of MMP-2, 9, TIMP-1, 2 and flk-1. J Korean Ophthalmol Soc. 2001; 42:1053–1062.11. Nordlund ML, Pepose JS. Corneal response to infection. In : Krachmer JH, Mannis MJ, Holland EJ, editors. Cornea. 2nd ed. Philadelphia: Elsevier;2005. p. 110.12. Chou E, Suzuma I, Way KJ, Opland D, Clermont AC, Naruse K, Suzuma K, Bowling NL, Vlahos CJ, Aiello LP, et al. Decreased cardiac expression of vascular endothelial growth factor and its receptors in insulin-resistant and diabetic States: a possible explanation for impaired collateral formation in cardiac tissue. Circulation. 2002; 105:373–379.13. Otani A, Takagi H, Suzuma K, Honda Y. Angiotensin II potentiates vascular endothelial growth factor-induced angiogenic activity in retinal microcapillary endothelial cells. Circ Res. 1998; 82:619–628.14. Fernandez LA, Twickler J, Mead A. Neovascularization produced by angiotensin II. J Lab Clin Med. 1985; 105:141–145.15. Jackson CJ, Jenkins KL. Type I collagen fibrils promote rapid vascular tube formation upon contact with the apical side of cultured endothelium. Exp Cell Res. 1991; 192:319–323.16. Schmidt G, Robenek H, Harrach B, Glössl J, Nolte V, Hörmann H, Richter H, Kresse H. Interaction of small dermatan sulfate proteoglycan from fibroblasts with fibronectin. J Cell Biol. 1987; 104:1683–1691.17. Schönherr E, Witsch-Prehm P, Harrach B, Robenek H, Rauterberg J, Kresse H. Interaction of biglycan with type I collagen. J Biol Chem. 1995; 270:2776–2783.18. Schönherr E, O'Connell BC, Schittny J, Robenek H, Fastermann D, Fisher LW, Plenz G, Vischer P, Young MF, Kresse H. Paracrine or virus-mediated induction of decorin expression by endothelial cells contributes to tube formation and prevention of apoptosis in collagen lattices. Eur J Cell Biol. 1999; 78:44–55.19. Nelimarkka L, Salminen H, Kuopio T, Nikkari S, Ekfors T, Laine J, Pelliniemi L, Järveläinen H. Decorin is produced by capillary endothelial cells in inflammation-associated angiogenesis. Am J Pathol. 2001; 158:345–353.20. Witmer AN, Vrensen GF, Van Noorden CJ, Schlingemann RO. Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retin Eye Res. 2003; 22:1–29.21. Jacobi J, Sydow K, von Degenfeld G, Zhang Y, Dayoub H, Wang B, Patterson AJ, Kimoto M, Blau HM, Cooke JP. Overexpression of dimethylarginine dimethylaminohydrolase reduces tissue asymmetric dimethylarginine levels and enhances angiogenesis. Circulation. 2005; 111:1431–1438.22. Ishikawa H, Heaney AP, Yu R, Horwitz GA, Melmed S. Human pituitary tumor-transforming gene induces angiogenesis. J Clin Endocrinol Metab. 2001; 86:867–874.23. Benjamin LE, Golijanin D, Itin A, Pode D, Keshet E. Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J Clin Invest. 1999; 103:159–165.24. Benjamin LE, Hemo I, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development. 1998; 125:1591–1598.25. Lindahl P, Johansson BR, Levéen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997; 277:242–245.26. Enge M, Bjarnegård M, Gerhardt H, Gustafsson E, Kalén M, Asker N, Hammes HP, Shani M, Fässler R, Betsholtz C. Endothelium-specific platelet-derived growth factor-B ablation mimics diabetic retinopathy. EMBO J. 2002; 21:4307–4316.27. Khachigian LM. Early growth response-1 in cardiovascular pathobiology. Circ Res. 2006; 98:186–191.28. Belperio JA, Keane MP, Arenberg DA, Addison CL, Ehlert JE, Burdick MD, Strieter RM. CXC chemokines in angiogenesis. J Leukoc Biol. 2000; 68:1–8.29. Kuo CT, Veselits ML, Barton KP, Lu MM, Clendenin C, Leiden JM. The LKLF transcription factor is required for normal tunica media formation and blood vessel stabilization during murine embryogenesis. Genes Dev. 1997; 11:2996–3006.30. Lee EW, Michalkiewicz M, Kitlinska J, Kalezic I, Switalska H, Yoo P, Sangkharat A, Ji H, Li L, Michalkiewicz T, et al. Neuropeptide Y induces ischemic angiogenesis and restores function of ischemic skeletal muscles. J Clin Invest. 2003; 111:1853–1862.31. Wang B, Sun J, Kitamoto S, Yang M, Grubb A, Chapman HA, Kalluri R, Shi GP. Cathepsin S controls angiogenesis and tumor growth via matrix-derived angiogenic factors. J Biol Chem. 2006; 281:6020–6029.32. Bussolati B, Ahmed A, Pemberton H, Landis RC, Di Carlo F, Haskard DO, Mason JC. Bifunctional role for VEGF-induced heme oxygenase-1 in vivo: induction of angiogenesis and inhibition of leukocytic infiltration. Blood. 2004; 103:761–766.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Gene Microarray Related with Apoptosis in Diabetic OLETF Keratocytes
- Microarray for Genes Associated with Signal Transduction in Diabetic OLETF Keratocytes
- In Silico Screening for Angiogenesis-Related Genes in Rat Astrocytes
- Adipose Gene Expression Profiles Related to Metabolic Syndrome Using Microarray Analyses in Two Different Models
- DNA Microarray-Based Gene Expression Profiling in Porcine Keratocytes and Corneal Endothelial Cells and Comparative Analysis Associated with Xeno-related Rejection