Lab Anim Res.
2015 Mar;31(1):1-6. 10.5625/lar.2015.31.1.1.
An age-dependent alteration of the respiratory exchange ratio in the db/db mouse
- Affiliations
-
- 1Laboratory Animal Resource Center, KRIBB, Cheongju, Korea. namk@kribb.re.kr
- 2Laboratory Animal Medicine, College of Veterinary Medicine, Chungbuk National University, Cheongju, Korea.
- KMID: 1787645
- DOI: http://doi.org/10.5625/lar.2015.31.1.1
Abstract
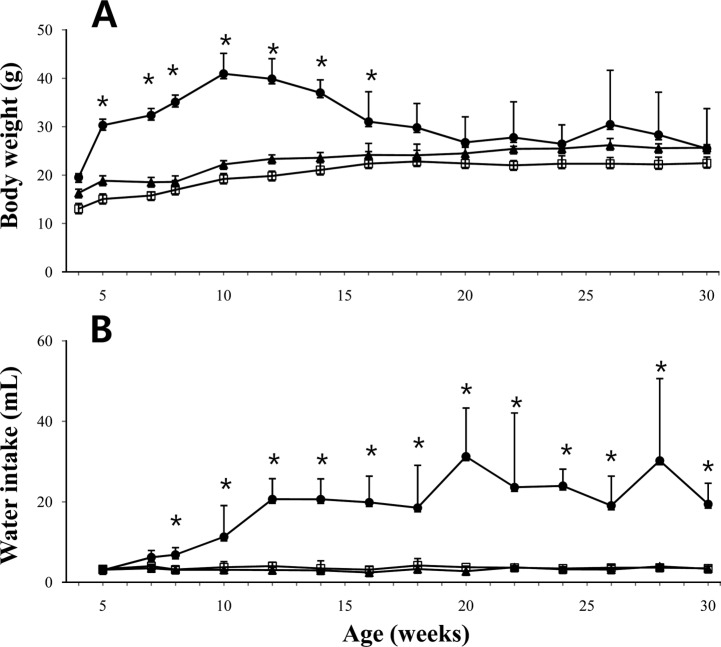
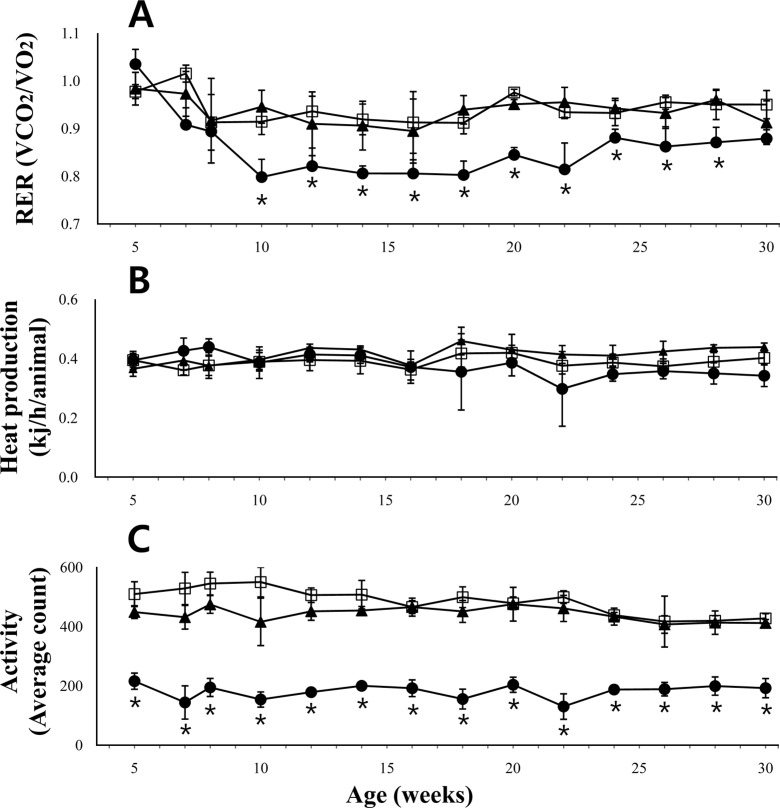
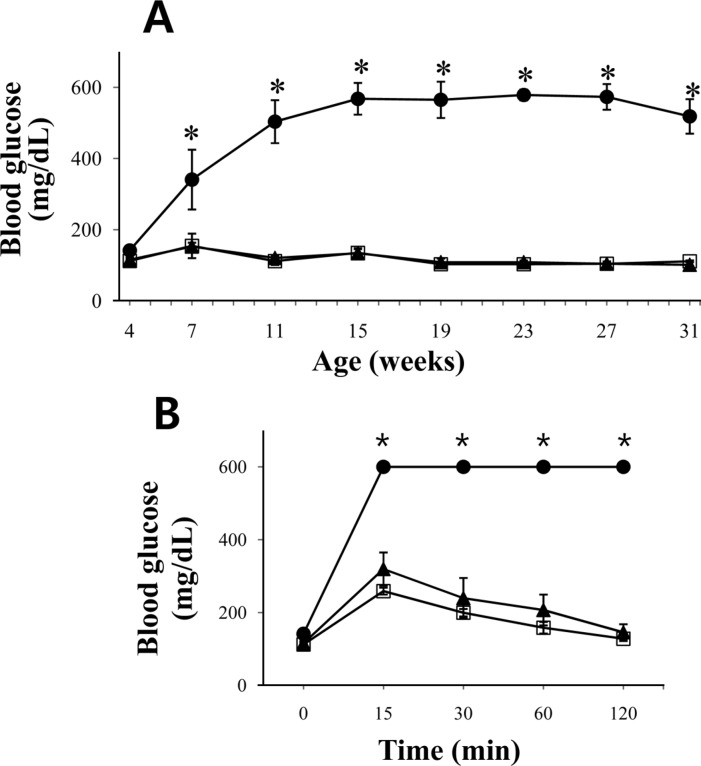
- The leptin receptor-deficient db/db mouse is a rodent model of type 2 diabetes and obesity. Diabetes in db/db mice shows an age-dependent progression, with early insulin resistance followed by an insulin secretory defect resulting in profound hyperglycemia. However, there is insufficient data on agedependent changes of energy metabolism in db/db mice. We demonstrated an age-dependent decrease in the respiratory exchange ratio (RER), calculated by a ratio of VO2/VCO2, in db/db mice. The RER determined by indirect calorimetry, was 1.03 in db/db mice under 6 weeks of age, which were similar to those in heterozygote (db/+) and wild-type (+/+) mice. However, RER decreased from approximately 0.9 to 0.8 by 10 weeks of age and subsequently returned to approximately 0.9 at 22 weeks of age. The changes in RER were concurrent with the alterations in body weight and blood glucose level. However, other metabolic indicators such as glucose tolerance, changes in body fat mass, and urinary glucose levels, did not change with age. The results suggested that the energy source utilized in db/db mice changed with the age-related progression of diabetes.
MeSH Terms
Figure
Reference
-
1. Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010; 87(1):4–14. PMID: 19896746.
Article2. Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014; 103(2):137–149. PMID: 24630390.
Article3. King AJ. The use of animal models in diabetes research. Br J Pharmacol. 2012; 166(3):877–894. PMID: 22352879.
Article4. Cohran VC, Bates MD. Leptin signaling and obesity: weight and see. Gastroenterology. 2003; 124(5):1546–1548. PMID: 15534982.
Article5. Lewis GF, Carpentier A, Adeli K, Giacca A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev. 2002; 23(2):201–229. PMID: 11943743.
Article6. Hummel KP, Dickie MM, Coleman DL. Diabetes, a new mutation in the mouse. Science. 1966; 153(3740):1127–1128. PMID: 5918576.
Article7. Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. Role of leptin in the neuroendocrine response to fasting. Nature. 1996; 382(6588):250–252. PMID: 8717038.
Article8. Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, Duyk GM, Tepper RI, Morgenstern JP. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996; 84(3):491–495. PMID: 8608603.
Article9. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994; 372(6505):425–432. PMID: 7984236.
Article10. Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995; 269(5223):540–543. PMID: 7624776.11. Heiman ML, Ahima RS, Craft LS, Schoner B, Stephens TW, Flier JS. Leptin inhibition of the hypothalamic-pituitary-adrenal axis in response to stress. Endocrinology. 1997; 138(9):3859–3863. PMID: 9275075.12. Brandi LS, Bertolini R, Calafa M. Indirect calorimetry in critically ill patients: clinical applications and practical advice. Nutrition. 1997; 13(4):349–358. PMID: 9178287.
Article13. Battezzati A, Viganò R. Indirect calorimetry and nutritional problems in clinical practice. Acta Diabetol. 2001; 38(1):1–5. PMID: 11487171.
Article14. Schwenk A, Meriläinen PT, Macallan DC. Indirect calorimetry in patients with active respiratory infection--prevention of cross-infection. Clin Nutr. 2002; 21(5):385–388. PMID: 12381335.15. GLK . Measurements in physical therapy by Jules M. Rothstein Clinics in Physical Therapy Vol. 7. Churchill Livingstone, 1985. £27.50. Clin Biomech (Bristol, Avon). 1987; 2(1):61.16. Osborn O, Sanchez-Alavez M, Brownell SE, Ross B, Klaus J, Dubins J, Beutler B, Conti B, Bartfai T. Metabolic characterization of a mouse deficient in all known leptin receptor isoforms. Cell Mol Neurobiol. 2010; 30(1):23–33. PMID: 19582570.
Article17. Li YY, Yu LF, Zhang LN, Qiu BY, Su MB, Wu F, Chen DK, Pang T, Gu M, Zhang W, Ma WP, Jiang HW, Li JY, Nan FJ, Li J. Novel small-molecule AMPK activator orally exerts beneficial effects on diabetic db/db mice. Toxicol Appl Pharmacol. 2013; 273(2):325–334. PMID: 24055643.
Article18. Muoio DM, Lynis Dohm G. Peripheral metabolic actions of leptin. Best Pract Res Clin Endocrinol Metab. 2002; 16(4):653–666. PMID: 12468413.
Article19. Harris RB, Mitchell TD, Yan X, Simpson JS, Redmann SM Jr. Metabolic responses to leptin in obese db/db mice are strain dependent. Am J Physiol Regul Integr Comp Physiol. 2001; 281(1):R115–R132. PMID: 11404285.
Article20. Trayhurn P, James WP. Thermoregulation and non-shivering thermogenesis in the genetically obese (ob/ob) mouse. Pflugers Arch. 1978; 373(2):189–193. PMID: 565045.
Article21. Lee SM, Bressler R. Prevention of diabetic nephropathy by diet control in the db/db mouse. Diabetes. 1981; 30(2):106–111. PMID: 7009265.
Article22. Edgel KA, McMillen TS, Wei H, Pamir N, Houston BA, Caldwell MT, Mai PO, Oram JF, Tang C, Leboeuf RC. Obesity and weight loss result in increased adipose tissue ABCG1 expression in db/db mice. Biochim Biophys Acta. 2012; 1821(3):425–434. PMID: 22179025.
Article23. Dittrich HM, Hahn von Dorsche H. [The anatomical and histological investigation of the pancreas in the 19th century and till the discovery of insulin (1921). 2. The pancreas research from the discovery of islets (1869) till the discovery of pancreasdiabetes (1889) (author's transl)]. Anat Anz. 1978; 143(3):231–241. PMID: 686388.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Rapid and efficient identification of the mouse leptin receptor mutation (C57BL/KsJ-db/db) by tetra-primer amplification refractory mutation system-polymerase chain reaction (ARMS-PCR) analysis
- Silk fibroin hydrolysate exerts an anti-diabetic effect by increasing pancreatic beta cell mass in C57BL/KsJ-db/db mice
- Daraesoon (shoot of hardy kiwi) mitigates hyperglycemia in db/db mice by alleviating insulin resistance and inflammation
- Effect of glucagon-like peptide 1 on salivary gland hypofunction in diabetic db/db mice
- Effects of transient threshold shift and ambient noise on sensitivity and specificity of first screening of special health examination for noise