J Vet Sci.
2012 Dec;13(4):339-344.
Silk fibroin hydrolysate exerts an anti-diabetic effect by increasing pancreatic beta cell mass in C57BL/KsJ-db/db mice
- Affiliations
-
- 1Department of Medical Genetics, College of Medicine, Hallym University, Chuncheon 200-702, Korea. jgsuh@hallym.ac.kr
- 2Department of Internal Medicine, College of Medicine, Hallym University, Chuncheon 200-702, Korea.
- 3Department of Biochemistry, College of Medicine, Hallym University, Chuncheon 200-702, Korea.
- 4Center for Efficacy Assessment and Development of Functional Foods and Drugs, Hallym University, Chuncheon 200-702, Korea.
- 5Institute of Natural Medicine, Hallym University, Chuncheon 200-702, Korea.
Abstract
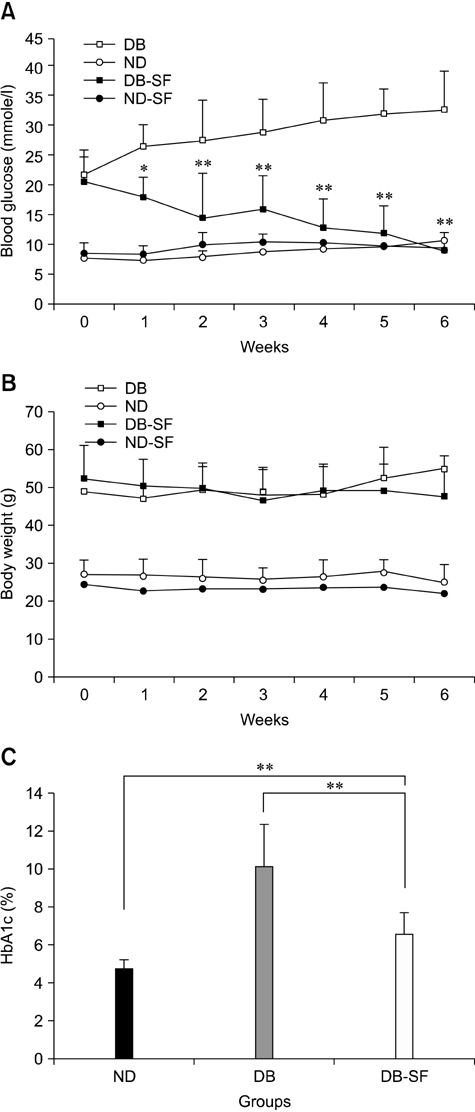
- Components of silk including silk fibroin have long been used as anti-diabetic remedies in oriental medicine. However, detailed mechanisms underlying these anti-diabetic effects remain unclear. In this study, we examined the anti-diabetic activity of silk fibroin hydrolysate (SFH) in C57BL/KsJ-db/db (db/db) mice, a well-known animal model of non-insulin dependent diabetes mellitus. When the db/db mice were administered SFH in drinking water for 6 weeks, hyperglycemia in the animals gradually disappeared and the level of glycosylated hemoglobin decreased, indicating that SFH plays important role in reducing the symptoms of diabetes. In addition, SFH-treated db/db mice exhibited improved glucose tolerance with increased plasma insulin levels. Immunohistochemical and morphological analyses showed that SFH up-regulated insulin production by increasing pancreatic beta cell mass in the mice. In summary, our results suggest that SFH exerts anti-diabetic effects by increasing pancreatic beta cell mass in a non-insulin dependent diabetes mellitus mouse model.
Keyword
MeSH Terms
Figure
Reference
-
1. Asano N, Oseki K, Tomioka E, Kizu H, Matsui K. N-containing sugars from Morus alba and their glycosidase inhibitory activities. Carbohydr Res. 1994. 259:243–255.
Article2. Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. β-cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003. 52:102–110.
Article3. Cheng AYY, Fantus IG. Oral antihyperglycemic therapy for type 2 diabetes mellitus. CMAJ. 2005. 172:213–226.
Article4. Drews G, Krippeit-Drews P, Düfer M. Oxidative stress and beta-cell dysfunction. Pflugers Arch. 2010. 460:703–718.
Article5. Fujimoto S. Therapeutic utility of biguanides in the treatment of NIDDM. Nihon Rinsho. 1999. 57:657–662.6. Gedulin BR, Nikoulina SE, Smith PA, Gedulin G, Nielsen LL, Baron AD, Parkes DG, Young AA. Exenatide (exendin-4) improves insulin sensitivity and β-cell mass in insulin-resistant obese fa/fa Zucker rats independent of glycemia and body weight. Endocrinology. 2005. 146:2069–2076.
Article7. Hikino H, Mizuno T, Oshima Y, Konno C. Isolation and hypoglycemic activity of moran A, a glycoprotein of Morus alba root barks. Planta Med. 1985. (2):159–160.8. Hoffmann IS, Roa M, Torrico F, Cubeddu LX. Ondansetron and metformin-induced gastrointestinal side effects. Am J Ther. 2003. 10:447–451.
Article9. Hyun CK, Kim IY, Frost SC. Soluble fibroin enhances insulin sensitivity and glucose metabolism in 3T3-L1 adipocytes. J Nutr. 2004. 134:3257–3263.
Article10. Joubert PH, Venter CP, Joubert HF, Hillebrand I. The effect of a 1-deoxynojirimycin derivative on post-prandial blood glucose and insulin levels in healthy black and white volunteers. Eur J Clin Pharmacol. 1985. 28:705–708.
Article11. Kim ED, Bayaraa T, Shin EJ, Hyun CK. Fibroin-derived peptides stimulate glucose transport in normal and insulin-resistant 3T3-L1 adipocytes. Biol Pharm Bull. 2009. 32:427–433.
Article12. Kim ES, Park SJ, Lee EJ, Kim BK, Huh H, Lee BJ. Purification and characterization of Moran 20K from Morus alba. Arch Pharm Res. 1999. 22:9–12.
Article13. Le Floch JP, Escuyer P, Baudin E, Baudon D, Perlemuter L. Blood glucose area under the curve. Methodological aspects. Diabetes Care. 1990. 13:172–175.
Article14. Lebovitz HE. Glipizide: a second-generation sulfonylurea hypoglycemic agent. Pharmacology, pharmacokinetics and clinical use. Pharmacotherapy. 1985. 5:63–77.
Article15. McCarthy MI. Genomics, type 2 diabetes, and obesity. N Engl J Med. 2010. 363:2339–2350.
Article16. Nahm JH, Oh YS. A study of pharmacological effect of silk fibroin. RDA J Agric Sci. 1995. 37:145–157.17. Nojima H, Kimura I, Chen FJ, Sugihara Y, Haruno M, Kato A, Asano N. Antihyperglycemic effects of N-containing sugars from Xanthocercis zambesiaca, Morus bombycis, Aglaonema treubii, and Castanospermum australe in streptozotocin-diabetic mice. J Nat Prod. 1998. 61:397–400.
Article18. Obatomi DK, Bikomo EO, Temple VJ. Anti-diabetic properties of the African mistletoe in streptozotocin-induced diabetic rats. J Ethnopharmacol. 1994. 43:13–17.
Article19. Park JH, Nam Y, Park SY, Kim JK, Choe NH, Lee JY, Oh YS, Suh JG. Silk fibroin has a protective effect against high glucose induced apoptosis in HIT-T15 cells. J Biochem Mol Toxicol. 2011. 25:238–243.
Article20. Rooman I, Lardon J, Bouwens L. Gastrin stimulates β-cell neogenesis and increases islet mass from transdifferentiated but not from normal exocrine pancreas tissue. Diabetes. 2002. 51:686–690.
Article21. Scheen AJ. Benefits and limitations of protein diets in obese patients with type 2 diabetes. Ann Endocrinol (Paris). 1999. 60:443–450.22. Taniguchi S, Asano N, Tomino F, Miwa I. Potentiation of glucose-induced insulin secretion by fagomine, a pseudo-sugar isolated from mulberry leaves. Horm Metab Res. 1998. 30:679–683.
Article23. Uebersax L, Merkle HP, Meinel L. Insulin-like growth factor I releasing silk fibroin scaffolds induce chondrogenic differentiation of human mesenchymal stem cells. J Control Release. 2008. 127:12–21.
Article24. Zhou F, Xue Z, Wang J. Antihypertensive effects of silk fibroin hydrolysate by alcalase and purification of an ACE inhibitory dipeptide. J Agric Food Chem. 2010. 58:6735–6740.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sargassum coreanum extract alleviates hyperglycemia and improves insulin resistance in db/db diabetic mice
- Effect of beta-glucan from Aureobasidium on dermal wound healing in diabetic C57BL/KsJ-db/db mouse model
- Gynura procumbens extract improves insulin sensitivity and suppresses hepatic gluconeogenesis in C57BL/KsJ-db/db mice
- Anti-diabetic effect of purple corn extract on C57BL/KsJ db/db mice
- Effects of Anti-Vascular Endothelial Growth Factor (VEGF) on Pancreatic Islets in Mouse Model of Type 2 Diabetes Mellitus