J Korean Med Sci.
2008 Jun;23(3):406-413. 10.3346/jkms.2008.23.3.406.
Atelectasis Induced by Thoracotomy Causes Lung Injury during Mechanical Ventilation in Endotoxemic Rats
- Affiliations
-
- 1Department of Medicine, Keimyung University Dongsan Hospital, Daegu, Korea. wichoi@dsmc.or.kr
- 2Department of Pathology, Keimyung University Dongsan Hospital, Daegu, Korea.
- 3Department of Anesthesiology, Keimyung University Dongsan Hospital, Daegu, Korea.
- 4Pulmonary/Critical Care Units, Department of Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, MA, U.S.A.
- 5Department of Pathology, Seoul National University Hospital, Seoul, Korea.
- KMID: 1786877
- DOI: http://doi.org/10.3346/jkms.2008.23.3.406
Abstract
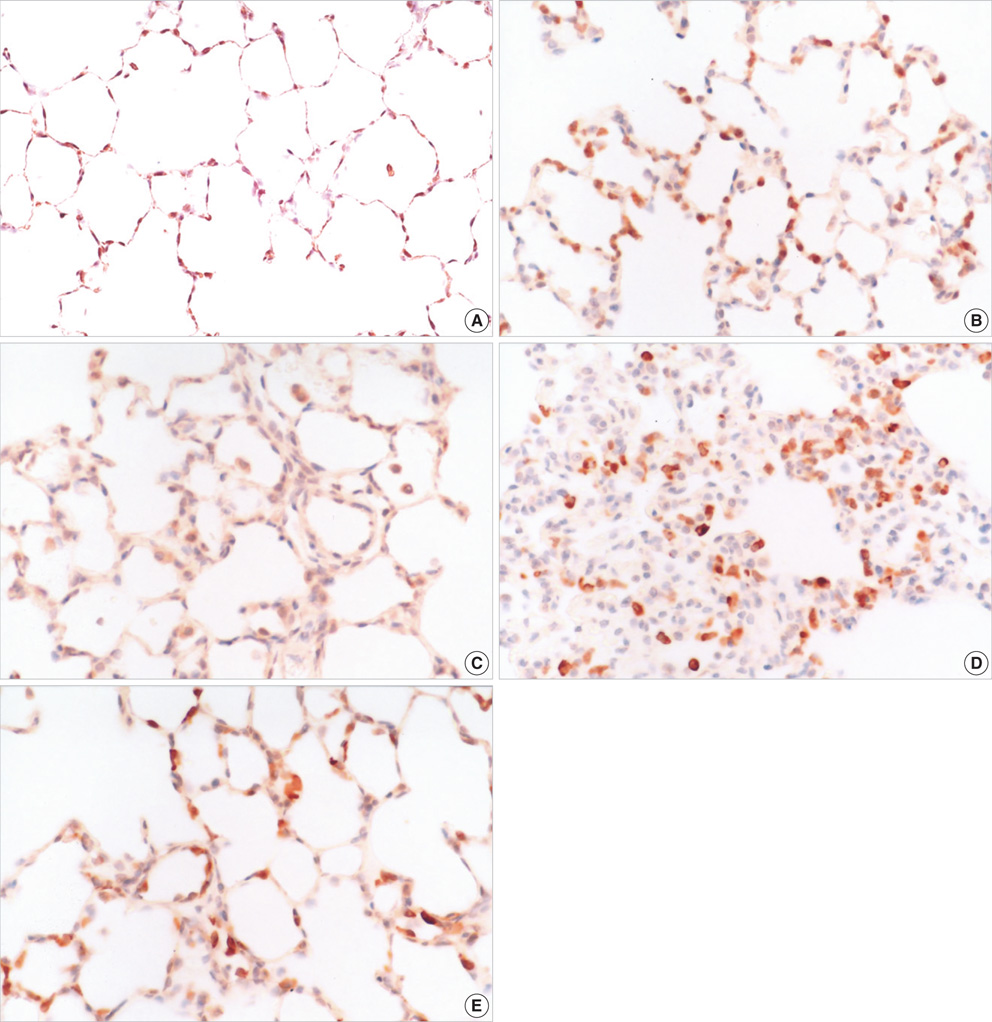
- Atelectasis can impair arterial oxygenation and decrease lung compliance. However, the effects of atelectasis on endotoxemic lungs during ventilation have not been well studied. We hypothesized that ventilation at low volumes below functional residual capacity (FRC) would accentuate lung injury in lipopolysaccharide (LPS)-pretreated rats. LPS-pretreated rats were ventilated with room air at 85 breaths/min for 2 hr at a tidal volume of 10 mL/kg with or without thoracotomy. Positive end-expiratory pressure (PEEP) was applied to restore FRC in the thoracotomy group. While LPS or thoracotomy alone did not cause significant injury, the combination of endotoxemia and thoracotomy caused significant hypoxemia and hypercapnia. The injury was observed along with a marked accumulation of inflammatory cells in the interstitium of the lungs, predominantly comprising neutrophils and mononuclear cells. Immunohistochemistry showed increased inducible nitric oxide synthase (iNOS) expression in mononuclear cells accumulated in the interstitium in the injury group. Pretreatment with PEEP or an iNOS inhibitor (1400 W) attenuated hypoxemia, hypercapnia, and the accumulation of inflammatory cells in the lung. In conclusion, the data suggest that atelectasis induced by thoracotomy causes lung injury during mechanical ventilation in endotoxemic rats through iNOS expression.
Keyword
MeSH Terms
-
Animals
Blood Pressure
Carbon Dioxide/blood
Cardiac Output
Combined Modality Therapy
Endotoxemia/*complications/immunology/pathology
Functional Residual Capacity
Immunohistochemistry
Leukocytes, Mononuclear/pathology
Lipopolysaccharides/pharmacology
Lung/enzymology/pathology/physiopathology
Lung Compliance
Lung Volume Measurements
Male
Neutrophils/pathology
Nitric Oxide Synthase Type II/metabolism
Oxygen/blood
Positive-Pressure Respiration/*adverse effects
Pulmonary Atelectasis/*etiology/pathology/*therapy
Rats
Rats, Sprague-Dawley
Thoracotomy/*adverse effects
Figure
Reference
-
1. Rehder K, Sessler AD, Marsh HM. General anesthesia and the lung. Am Rev Respir Dis. 1975. 112:541–563.2. Lindberg P, Gunnarsson L, Tokics L, Secher E, Lundquist H, Brismar B, Hedenstierna G. Atelectasis and lung function in the postoperative period. Acta Anaesthesiol Scand. 1992. 36:546–553.
Article3. Reissmann H, Bohm SH, Suarez-Sipmann F, Tusman G, Buschmann C, Maisch S, Pesch T, Thamm O, Plumers C, Schulte am Esch J, Hedenstierna G. Suctioning through a double-lumen endotracheal tube helps to prevent alveolar collapse and to preserve ventilation. Intensive Care Med. 2005. 31:431–440.
Article4. Muller JM, Erasmi H, Stelzner M, Zieren U, Pichlmaier H. Surgical therapy of oesophageal carcinoma. Br J Surg. 1990. 77:845–857.5. Tandon S, Batchelor A, Bullock R, Gascoigne A, Griffin M, Hayes N, Hing J, Shaw I, Warnell I, Baudouin SV. Peri-operative risk factors for acute lung injury after elective oesophagectomy. Br J Anaesth. 2001. 86:633–638.6. Taskar V, John J, Evander E, Robertson B, Jonson B. Surfactant dysfunction makes lungs vulnerable to repetitive collapse and reexpansion. Am J Respir Crit Care Med. 1997. 155:313–320.
Article7. Neumann P, Berglund JE, Mondejar EF, Magnusson A, Hedenstierna G. Effect of different pressure levels on the dynamics of lung collapse and recruitment in oleic-acid-induced lung injury. Am J Respir Crit Care Med. 1998. 158:1636–1643.
Article8. Steinberg JM, Schiller HJ, Halter JM, Gatto LA, Lee HM, Pavone LA, Nieman GF. Alveolar instability causes early ventilator-induced lung injury independent of neutrophils. Am J Respir Crit Care Med. 2004. 169:57–63.
Article9. Woo SW, Hedley-Whyte J. Macrophage accumulation and pulmonary edema due to thoracotomy and lung over inflation. J Appl Physiol. 1972. 33:14–21.
Article10. Tremblay L, Valenza F, Ribeiro SP, Li J, Slutsky AS. Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J Clin Invest. 1997. 99:944–952.
Article11. Quinn DA, Moufarrej RK, Volokhov A, Hales CA. Interactions of lung stretch, hyperoxia, and MIP-2 production in ventilator-induced lung injury. J Appl Physiol. 2002. 93:517–525.12. Gosgnach W, Messika-Zeitoun D, Gonzalez W, Philipe M, Michel JB. Shear stress induces iNOS expression in cultured smooth muscle cells: role of oxidative stress. Am J Physiol Cell Physiol. 2000. 279:C1880–C1888.
Article13. Kristof AS, Goldberg P, Laubach V, Hussain SN. Role of inducible nitric oxide synthase in endotoxin-induced acute lung injury. Am J Respir Crit Care Med. 1998. 158:1883–1889.
Article14. Frank JA, Pittet JF, Lee H, Godzich M, Matthay MA. High tidal volume ventilation induces NOS2 and impairs cAMP-dependent air space fluid clearance. Am J Physiol Lung Cell Mol Physiol. 2003. 284:L791–L798.15. Peng X, Abdulnour RE, Sammani S, Ma SF, Han EJ, Hasan EJ, Tuder R, Garcia JG, Hassoun PM. Inducible nitric oxide synthase contributes to ventilator-induced lung injury. Am J Respir Crit Care Med. 2005. 172:470–479.
Article16. Lai YL, Hildebrandt J. Respiratory mechanics in the anesthetized rat. J Appl Physiol. 1978. 45:255–260.
Article17. Wohl ME, Turner J, Mead J. Static volume-pressure curves of dog lungs-in vivo and in vitro. J Appl Physiol. 1968. 24:348–354.
Article18. Garvey EP, Oplinger JA, Furfine ES, Kiff RJ, Laszlo F, Whittle BJ, Knowles RG. 1400 W is a slow, tight binding and highly selective inhibitor of inducible nitric-oxide synthase in vitro and in vivo. J Biol Chem. 1997. 272:4959–4963.19. Thomsen LL, Scott JM, Topley P, Knowles RG, Keerie AJ, Frend AJ. Selective inhibition of inducible nitric oxide synthase inhibits tumor growth in vivo: studies with 1400 W, a novel inhibitor. Cancer Res. 1997. 57:3300–3304.20. Krieglstein CF, Cerwinka WH, Laroux FS, Salter JW, Russell JM, Schuermann G, Grisham MB, Ross CR, Granger DN. Regulation of murine intestinal inflammation by reactive metabolites of oxygen and nitrogen: divergent roles of superoxide and nitric oxide. J Exp Med. 2001. 194:1207–1218.21. van Kaam AH, Lachmann RA, Herting E, De Jaegere A, van Iwaarden F, Noorduyn LA, Kok JH, Haitsma JJ, Lachmann B. Reducing atelectasis attenuates bacterial growth and translocation in experimental pneumonia. Am J Respir Crit Care Med. 2004. 169:1046–1053.
Article22. The acute respiratory distress syndrome network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000. 342:1301–1308.23. Wongsurakiat P, Pierson DJ, Rubenfeld GD. Changing pattern of ventilator settings in patients without acute lung injury: changes over 11 years in a single institution. Chest. 2004. 126:1281–1291.24. Dreyfuss D, Saumon G. Role of tidal volume, FRC, and end-inspiratory volume in the development of pulmonary edema following mechanical ventilation. Am Rev Respir Dis. 1993. 148:1194–1203.
Article25. Muscedere JG, Mullen JB, Gan K, Slutsky AS. Tidal ventilation at low airway pressures can augment lung injury. Am J Respir Crit Care Med. 1994. 149:1327–1334.
Article26. Enhorning G, Holm BA. Disruption of pulmonary surfactant's ability to maintain openness of a narrow tube. J Appl Physiol. 1993. 74:2922–2927.
Article27. Enhorning G, Duffy LC, Welliver RC. Pulmonary surfactant maintains patency of conducting airways in the rat. Am J Respir Crit Care Med. 1995. 151:554–556.
Article28. Kawano T, Mori S, Cybulsky M, Burger R, Ballin A, Cutz E, Bryan AC. Effect of granulocyte depletion in a ventilated surfactant-depleted lung. J Appl Physiol. 1987. 62:27–33.
Article29. Razavi HM, Wang le F, Weicker S, Rohan M, Law C, McCormack DG, Mehta S. Pulmonary neutrophil infiltration in murine sepsis: role of inducible nitric oxide synthase. Am J Respir Crit Care Med. 2004. 170:227–233.30. Numata M, Suzuki S, Miyazawa N, Miyashita A, Nagashima Y, Inoue S, Kaneko T, Okubo T. Inhibition of inducible nitric oxide synthase prevents LPS-induced acute lung injury in dogs. J Immunol. 1998. 160:3031–3037.31. Fowler AA, Hamman RF, Good JT, Benson KN, Baird M, Eberle DJ, Petty TL, Hyers TM. Adult respiratory distress syndrome: risk with common predispositions. Ann Intern Med. 1983. 98:593–597.
Article32. Hudson LD, Milberg JA, Anardi D, Maunder RJ. Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1995. 151:293–301.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ventilator-Induced Lung Injury
- Collagen Synthesis in an in Vivo Rat Model of Ventilator-induced Lung Injury
- Brain and Lung: Lung Injury in Patients with Brain Injury
- Ventilator Induced Lung Injury (VILI) and Strategies to Minimize the Injury
- Treatment of acute respiratory failure: invasive mechanical ventilation