J Neurocrit Care.
2017 Jun;10(1):1-6. 10.18700/jnc.170009.
Brain and Lung: Lung Injury in Patients with Brain Injury
- Affiliations
-
- 1Department of Intensive Care Medicine and Neurology, Stroke Center, Dong-A University Hospital, Busan, Korea. nr.jungjh@gmail.com
- KMID: 2385879
- DOI: http://doi.org/10.18700/jnc.170009
Abstract
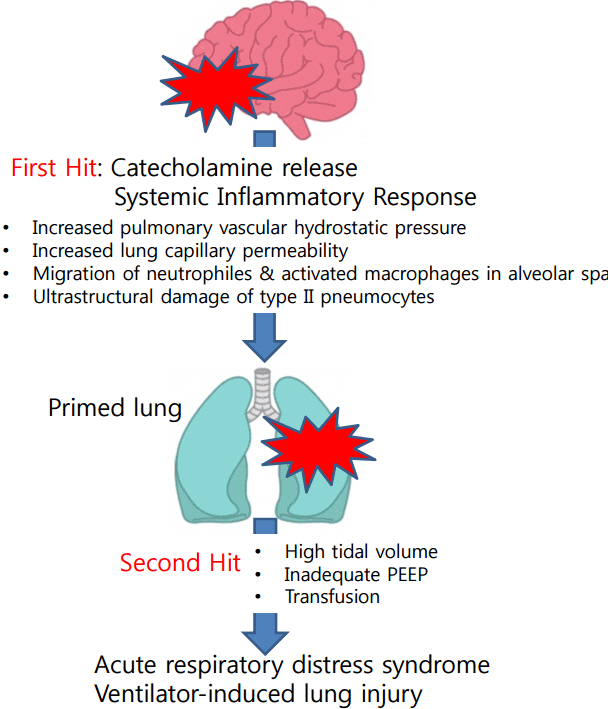
- Neurocritically ill patients are at an increased risk of other organ dysfunctions, especially lung injury. Major pulmonary complications, including acute respiratory distress syndrome, ventilator-associated pneumonia, and neurogenic pulmonary edema, are frequently caused by brain injury, and are associated with poor outcome. Brain and lung have strong interactions via complex pathways from the brain to the lung, and vice versa. Excessive release of catecholamines and systemic inflammatory responses play an integral role in the development of pulmonary dysfunction after brain injuries. Mechanical ventilation is commonly used to manage pulmonary dysfunctions associated with brain injury, and lung protective ventilation strategies reduce injuries to the lung and brain. This review focuses on the current knowledge regarding the epidemiology and pathophysiology of lung injuries in patients with neurocritical illness, and the various strategies of mechanical ventilation used to reduce lung injury.
MeSH Terms
Figure
Cited by 2 articles
-
Monitoring and Interpretation of Mechanical Ventilator Waveform in the Neuro-Intensive Care Unit
Jin Park
J Neurocrit Care. 2018;11(2):63-70. doi: 10.18700/jnc.180069.Oxygen supplementation via high-flow nasal cannula is an effective treatment for pneumocephalus
Deok-Soo Lee, Jae-Kwan Cha, Dong Hyun Lee, Ki Sup Byun, Jin-Heon Jeong
J Neurocrit Care. 2019;12(2):98-101. doi: 10.18700/jnc.190095.
Reference
-
1. Mascia L. Acute lung injury in patients with severe brain injury: a double hit model. Neurocrit Care. 2009; 11:417–26.
Article2. Pelosi P, Rocco PR. The lung and the brain: a dangerous cross-talk. Crit Care. 2011; 15:168.
Article3. Mrozek S, Constantin JM, Geeraerts T. Brain-lung crosstalk: implications for neurocritical care patients. World J Crit Care Med. 2015; 4:163–78.
Article4. Blanch L, Quintel M. Lung-brain cross talk in the critically ill. Intensive Care Med. 2017; 43:557–9.
Article5. Stocchetti N, Furlan A, Volta F. Hypoxemia and arterial hypotension at the accident scene in head injury. J Trauma. 1996; 40:764–7.
Article6. Bratton SL, Davis RL. Acute lung injury in isolated traumatic brain injury. Neurosurgery. 1997; 40:707–12. ; discussion 12.
Article7. Contant CF, Valadka AB, Gopinath SP, Hannay HJ, Robertson CS. Adult respiratory distress syndrome: a complication of induced hypertension after severe head injury. J Neurosurg. 2001; 95:560–8.
Article8. Holland MC, Mackersie RC, Morabito D, Campbell AR, Kivett VA, Patel R, et al. The development of acute lung injury is associated with worse neurologic outcome in patients with severe traumatic brain injury. J Trauma. 2003; 55:106–11.
Article9. Roch A, Michelet P, Jullien AC, Thirion X, Bregeon F, Papazian L, et al. Long-term outcome in intensive care unit survivors after mechanical ventilation for intracerebral hemorrhage. Crit Care Med. 2003; 31:2651–6.
Article10. Zygun DA, Kortbeek JB, Fick GH, Laupland KB, Doig CJ. Nonneurologic organ dysfunction in severe traumatic brain injury. Crit Care Med. 2005; 33:654–60.
Article11. Kahn JM, Caldwell EC, Deem S, Newell DW, Heckbert SR, Rubenfeld GD. Acute lung injury in patients with subarachnoid hemorrhage: incidence, risk factors, and outcome. Crit Care Med. 2006; 34:196–202.
Article12. Wartenberg KE, Schmidt JM, Claassen J, Temes RE, Frontera JA, Ostapkovich N, et al. Impact of medical complications on outcome after subarachnoid hemorrhage. Crit Care Med. 2006; 34:617–23. ; quiz 624.
Article13. Maramattom BV, Weigand S, Reinalda M, Wijdicks EF, Manno EM. Pulmonary complications after intracerebral hemorrhage. Neurocrit Care. 2006; 5:115–9.
Article14. Mascia L, Zavala E, Bosma K, Pasero D, Decaroli D, Andrews P, et al. High tidal volume is associated with the development of acute lung injury after severe brain injury: an international observational study. Crit Care Med. 2007; 35:1815–20.
Article15. Pelosi P, Ferguson ND, Frutos-Vivar F, Anzueto A, Putensen C, Raymondos K, et al. Management and outcome of mechanically ventilated neurologic patients. Crit Care Med. 2011; 39:1482–92.
Article16. Elmer J, Hou P, Wilcox SR, Chang Y, Schreiber H, Okechukwu I, et al. Acute respiratory distress syndrome after spontaneous intracerebral hemorrhage*. Crit Care Med. 2013; 41:1992–2001.
Article17. Rincon F, Maltenfort M, Dey S, Ghosh S, Vibbert M, Urtecho J, et al. The prevalence and impact of mortality of the acute respiratory distress syndrome on admissions of patients with ischemic stroke in the United States. J Intensive Care Med. 2014; 29:357–64.
Article18. Solenski NJ, Haley EC Jr, Kassell NF, Kongable G, Germanson T, Truskowski L, et al. Medical complications of aneurysmal subarachnoid hemorrhage: a report of the multicenter, cooperative aneurysm study. Participants of the Multicenter Cooperative Aneurysm Study. Crit Care Med. 1995; 23:1007–17.19. Hoesch RE, Lin E, Young M, Gottesman RF, Altaweel L, Nyquist PA, et al. Acute lung injury in critical neurological illness. Crit Care Med. 2012; 40:587–93.
Article20. Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994; 149(3 Pt 1):818–24.
Article21. ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012; 307:2526–33.22. Kollef MH, Morrow LE, Niederman MS, Leeper KV, Anzueto A, Benz-Scott L, et al. Clinical characteristics and treatment patterns among patients with ventilator-associated pneumonia. Chest. 2006; 129:1210–8.
Article23. Woratyla SP, Morgan AS, Mackay L, Bernstein B, Barba C. Factors associated with early onset pneumonia in the severely brain-injured patient. Conn Med. 1995; 59:643–7.24. Bronchard R, Albaladejo P, Brezac G, Geffroy A, Seince PF, Morris W, et al. Early onset pneumonia: risk factors and consequences in head trauma patients. Anesthesiology. 2004; 100:234–9.25. Lepelletier D, Roquilly A, Demeure dit latte D, Mahe PJ, Loutrel O, Champin P, et al. Retrospective analysis of the risk factors and pathogens associated with early-onset ventilator- associated pneumonia in surgical-ICU head-trauma patients. J Neurosurg Anesthesiol. 2010; 22:32–7.26. Frontera JA, Fernandez A, Schmidt JM, Claassen J, Wartenberg KE, Badjatia N, et al. Impact of nosocomial infectious complications after subarachnoid hemorrhage. Neurosurgery. 2008; 62:80–7. ; discussion 87.
Article27. Cinotti R, Dordonnat-Moynard A, Feuillet F, Roquilly A, Rondeau N, Lepelletier D, et al. Risk factors and pathogens involved in early ventilator-acquired pneumonia in patients with severe subarachnoid hemorrhage. Eur J Clin Microbiol Infect Dis. 2014; 33:823–30.
Article28. Dziedzic T, Slowik A, Szczudlik A. Nosocomial infections and immunity: lesson from brain-injured patients. Crit Care. 2004; 8:266–70.29. Davison DL, Terek M, Chawla LS. Neurogenic pulmonary edema. Crit Care. 2012; 16:212.
Article30. Busl KM, Bleck TP. Neurogenic Pulmonary Edema. Crit Care Med. 2015; 43:1710–5.
Article31. Ochiai H, Yamakawa Y, Kubota E. Deformation of the ventrolateral medulla oblongata by subarachnoid hemorrhage from ruptured vertebral artery aneurysms causes neurogenic pulmonary edema. Neurol Med Chir (Tokyo). 2001; 41:529–34. ; discussion 534-5.
Article32. Friedman JA, Pichelmann MA, Piepgras DG, McIver JI, Toussaint LG 3rd, McClelland RL, et al. Pulmonary complications of aneurysmal subarachnoid hemorrhage. Neurosurgery. 2003; 52:1025–31. ; discussion 1031-2.
Article33. Rogers FB, Shackford SR, Trevisani GT, Davis JW, Mackersie RC, Hoyt DB. Neurogenic pulmonary edema in fatal and nonfatal head injuries. J Trauma. 1995; 39:860–6. ; discussion 866-8.
Article34. Heuer JF, Pelosi P, Hermann P, Perske C, Crozier TA, Bruck W, et al. Acute effects of intracranial hypertension and ARDS on pulmonary and neuronal damage: a randomized experimental study in pigs. Intensive Care Med. 2011; 37:1182–91.
Article35. Inamasu J, Sugimoto K, Yamada Y, Ganaha T, Ito K, Watabe T, et al. The role of catecholamines in the pathogenesis of neurogenic pulmonary edema associated with subarachnoid hemorrhage. Acta Neurochir (Wien). 2012; 154:2179–84. ; discussion 2184-5.
Article36. Avlonitis VS, Fisher AJ, Kirby JA, Dark JH. Pulmonary transplantation: the role of brain death in donor lung injury. Transplantation. 2003; 75:1928–33.
Article37. Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br J Pharmacol. 2006; 147 Suppl 1:S232–40.
Article38. Scholz M, Cinatl J, Schadel-Hopfner M, Windolf J. Neutrophils and the blood-brain barrier dysfunction after trauma. Med Res Rev. 2007; 27:401–16.
Article39. Lopez-Aguilar J, Villagra A, Bernabe F, Murias G, Piacentini E, Real J, et al. Massive brain injury enhances lung damage in an isolated lung model of ventilator-induced lung injury. Crit Care Med. 2005; 33:1077–83.40. Ranieri VM, Suter PM, Tortorella C, De Tullio R, Dayer JM, Brienza A, et al. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1999; 282:54–61.
Article41. Ranieri VM, Giunta F, Suter PM, Slutsky AS. Mechanical ventilation as a mediator of multisystem organ failure in acute respiratory distress syndrome. JAMA. 2000; 284:43–4.
Article42. Acute Respiratory Distress Syndrome Network, Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000; 342:1301–8.
Article43. Serpa Neto A, Cardoso SO, Manetta JA, Pereira VG, Esposito DC, Pasqualucci Mde O, et al. Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: a meta-analysis. JAMA. 2012; 308:1651–9.44. Lopez-Aguilar J, Fernandez-Gonzalo MS, Turon M, Quilez ME, Gomez-Simon V, Jodar MM, et al. [Lung-brain interaction in the mechanically ventilated patient]. Med Intensiva. 2013; 37:485–92.45. Huseby JS, Luce JM, Cary JM, Pavlin EG, Butler J. Effects of positive end-expiratory pressure on intracranial pressure in dogs with intracranial hypertension. J Neurosurg. 1981; 55:704–5.
Article46. McGuire G, Crossley D, Richards J, Wong D. Effects of varying levels of positive end-expiratory pressure on intracranial pressure and cerebral perfusion pressure. Crit Care Med. 1997; 25:1059–62.
Article47. Caricato A, Conti G, Della Corte F, Mancino A, Santilli F, Sandroni C, et al. Effects of PEEP on the intracranial system of patients with head injury and subarachnoid hemorrhage: the role of respiratory system compliance. J Trauma. 2005; 58:571–6.
Article48. Muench E, Bauhuf C, Roth H, Horn P, Phillips M, Marquetant N, et al. Effects of positive end-expiratory pressure on regional cerebral blood flow, intracranial pressure, and brain tissue oxygenation. Crit Care Med. 2005; 33:2367–72.
Article