J Korean Med Sci.
2011 Jun;26(6):740-746. 10.3346/jkms.2011.26.6.740.
Determination of Malignant and Invasive Predictors in Branch Duct Type Intraductal Papillary Mucinous Neoplasms of the Pancreas: A Suggested Scoring Formula
- Affiliations
-
- 1Department of Surgery, and Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea. jangjy4@snu.ac.kr
- 2Department of Surgery, Kyungpook National University College of Medicine, Daegu, Korea.
- 3Department of Surgery, Center for Liver Cancer, National Cancer Center, Goyang, Korea.
- 4Department of Surgery, Keimyung University School of Medicine, Daegu, Korea.
- 5Department of Surgery, Graduate School of Medicine, Pusan National University, Busan, Korea.
- 6Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 7Department of Surgery, Soonchunhyang University School of Medicine, Seoul, Korea.
- 8Department of Surgery, College of Medicine, Yeungnam University, Daegu, Korea.
- 9Department of Surgery, Chonnam National University Medical School, Gwangju, Korea.
- 10Department of Surgery, Chonbuk National University Medical School, Jeonju, Korea.
- 11Department of Surgery, College of Medicine, Chungnam National University, Daejeon, Korea.
- KMID: 1785958
- DOI: http://doi.org/10.3346/jkms.2011.26.6.740
Abstract
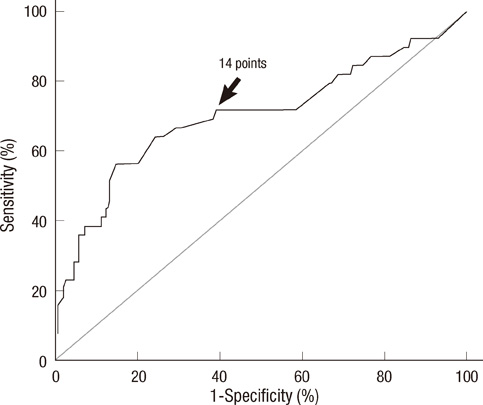
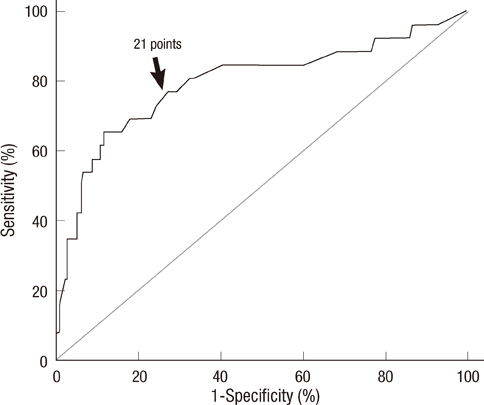
- Prediction of malignancy or invasiveness of branch duct type intraductal papillary mucinous neoplasm (Br-IPMN) is difficult, and proper treatment strategy has not been well established. The authors investigated the characteristics of Br-IPMN and explored its malignancy or invasiveness predicting factors to suggest a scoring formula for predicting pathologic results. From 1994 to 2008, 237 patients who were diagnosed as Br-IPMN at 11 tertiary referral centers in Korea were retrospectively reviewed. The patients' mean age was 63.1 +/- 9.2 yr. One hundred ninty-eight (83.5%) patients had nonmalignant IPMN (81 adenoma, 117 borderline atypia), and 39 (16.5%) had malignant IPMN (13 carcinoma in situ, 26 invasive carcinoma). Cyst size and mural nodule were malignancy determining factors by multivariate analysis. Elevated CEA, cyst size and mural nodule were factors determining invasiveness by multivariate analysis. Using the regression coefficient for significant predictors on multivariate analysis, we constructed a malignancy-predicting scoring formula: 22.4 (mural nodule [0 or 1]) + 0.5 (cyst size [mm]). In invasive IPMN, the formula was expressed as invasiveness-predicting score = 36.6 (mural nodule [0 or 1]) + 32.2 (elevated serum CEA [0 or 1]) + 0.6 (cyst size [mm]). Here we present a scoring formula for prediction of malignancy or invasiveness of Br-IPMN which can be used to determine a proper treatment strategy.
Keyword
MeSH Terms
-
Adenocarcinoma, Mucinous/*pathology
Adult
Aged
Aged, 80 and over
Carcinoembryonic Antigen/blood
Carcinoma, Pancreatic Ductal/*pathology
Carcinoma, Papillary/*pathology
Female
Humans
Magnetic Resonance Imaging
Male
Middle Aged
Multivariate Analysis
Neoplasm Invasiveness
Neoplasm Staging
Pancreatic Neoplasms/*pathology
Predictive Value of Tests
ROC Curve
Tomography, X-Ray Computed
Figure
Reference
-
1. Sohn TA, Yeo CJ, Cameron JL, Hruban RH, Fukushima N, Campbell KA, Lillemoe KD. Intraductal papillary mucinous neoplasms of the pancreas: an updated experience. Ann Surg. 2004. 239:788–797.2. Longnecker DS, Adsay NV, Fernandez-del Castillo C, Hruban RH, Kasugai T, Klimstra DS, Kloppel G, Luttges J, Memoli VA, Tosteson TD, Yanagisawa A, Wilentz R, Zamboni G. Histopathological diagnosis of pancreatic intraepithelial neoplasia and intraductal papillary-mucinous neoplasms: interobserver agreement. Pancreas. 2005. 31:344–349.3. Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, Yamaguchi K, Yamao K, Matsuno S. International Association of Pancreatology. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006. 6:17–32.4. Jang JY, Kim SW, Lee SE, Yang SH, Lee KU, Lee YJ, Kim SC, Han DJ, Choi DW, Choi SH, Heo JS, Cho BH, Yu HC, Yoon DS, Lee WJ, Lee HE, Kang GH, Lee JM. Treatment guidelines for branch duct type intraductal papillary mucinous neoplasms of the pancreas: when can we operate or observe? Ann Surg Oncol. 2008. 15:199–205.5. Schmidt CM, White PB, Waters JA, Yiannoutsos CT, Cummings OW, Baker M, Howard TJ, Zyromski NJ, Nakeeb A, DeWitt JM, Akisik FM, Sherman S, Pitt HA, Lillemoe KD. Intraductal papillary mucinous neoplasms: predictors of malignant and invasive pathology. Ann Surg. 2007. 246:644–651.6. Salvia R, Fernandez-del Castillo C, Bassi C, Thayer SP, Falconi M, Mantovani W, Pederzoli P, Warshaw AL. Main-duct intraductal papillary mucinous neoplasms of the pancreas: clinical predictors of malignancy and long-term survival following resection. Ann Surg. 2004. 239:678–685.7. Jang JY, Kim SW, Ahn YJ, Yoon YS, Choi MG, Lee KU, Han JK, Kim WH, Lee YJ, Kim SC, Han DJ, Kim YI, Choi SH, Cho BH, Yu HC, Yoon DS, Lee WJ, Lee KB, Kim YC, Lee KS, Kim MW, Kim HJ, Park YH. Multicenter analysis of clinicopathologic features of intraductal papillary mucinous tumor of the pancreas: is it possible to predict the malignancy before surgery? Ann Surg Oncol. 2005. 12:124–132.8. Paik KY, Choi SH. Experience of limited pancreatic head resection for management of branch duct intraductal papillary mucinous neoplasm in a single center. World J Gastroenterol. 2009. 15:2904–2907.9. Hwang DW, Jang JY, Lee SE, Lim CS, Lee KU, Kim SW. Clinicopathologic analysis of surgically proven intraductal papillary mucinous neoplasms of the pancreas in SNUH: a 15-year experience at a single academic institution. Langenbecks Arch Surg. 2010. doi: 10.1007/s00423-010-0674-6.10. Waters JA, Schmidt CM, Pinchot JW, White PB, Cummings OW, Pitt HA, Sandrasegaran K, Akisik F, Howard TJ, Nakeeb A, Zyromski NJ, Lillemoe KD. CT vs MRCP: optimal classification of IPMN type and extent. J Gastrointest Surg. 2008. 12:101–109.11. Sahani DV, Kadavigere R, Blake M, Fernandez-Del Castillo C, Lauwers GY, Hahn PF. Intraductal papillary mucinous neoplasm of pancreas: multi-detector row CT with 2D curved reformations--correlation with MRCP. Radiology. 2006. 238:560–569.12. Chiu SS, Lim JH, Lee WJ, Chang KT, Oh DK, Lee KT, Lee JK, Choi SH. Intraductal papillary mucinous tumour of the pancreas: differentiation of malignancy and benignancy by CT. Clin Radiol. 2006. 61:776–783.13. Moons KG, Harrell FE, Steyerberg EW. Should scoring rules be based on odds ratios or regression coefficients? J Clin Epidemiol. 2002. 55:1054–1055.14. Ohashi K, Murakami Y, Murayama M, Taketoshi T, Ohta T, Ohashi I. Four cases of mucin-producing cancer of the pancreas on specific findings of the papilla of Vater. Prog Dig Endosc. 1982. 20:348–351.15. Kubo H, Chijiiwa Y, Akahoshi K, Hamada S, Harada N, Sumii T, Takashima M, Nawata H. Intraductal papillary-mucinous tumors of the pancre as: differential diagnosis between benign and malignant tumors by endoscopic ultrasonography. Am J Gastroenterol. 2001. 96:1429–1434.16. Kawamoto S, Lawler LP, Horton KM, Eng J, Hruban RH, Fishman EK. MDCT of intraductal papillary mucinous neoplasm of the pancreas: evaluation of features predictive of invasive carcinoma. AJR Am J Roentgenol. 2006. 186:687–695.17. Okabayashi T, Kobayashi M, Nishimori I, Sugimoto T, Namikawa T, Okamoto K, Okamoto N, Kosaki T, Onishi S, Araki K. Clinicopathological features and medical management of intraductal papillary mucinous neoplasms. J Gastroenterol Hepatol. 2006. 21:462–467.18. Wiesenauer CA, Schmidt CM, Cummings OW, Yiannoutsos CT, Howard TJ, Wiebke EA, Goulet RJ Jr, McHenry L, Sherman S, Lehman GA, Cramer H, Madura JA. Preoperative predictors of malignancy in pancreatic intraductal papillary mucinous neoplasms. Arch Surg. 2003. 138:610–617.19. Kuroki T, Tajima Y, Tsutsumi R, Mishima T, Kitasato A, Adachi T, Kanematsu T. Inferior branch-preserving superior head resection of the pancreas with gastric wall-covering method for intraductal papillary mucinous adenoma. Am J Surg. 2006. 191:823–826.20. Yamao K, Ohashi K, Nakamura T, Suzuki T, Shimizu Y, Nakamura Y, Horibe Y, Yanagisawa A, Nakao A, Nimuara Y, Naito Y, Hayakawa T. The prognosis of intraductal papillary mucinous tumors of the pancreas. Hepatogastroenterology. 2000. 47:1129–1134.21. Bernard P, Scoazec JY, Joubert M, Kahn X, Le Borgne J, Berger F, Partensky C. Intraductal papillary-mucinous tumors of the pancreas: predictive criteria of malignancy according to pathological examination of 53 cases. Arch Surg. 2002. 137:1274–1278.22. Salvia R, Crippa S, Falconi M, Bassi C, Guarise A, Scarpa A, Pederzoli P. Branch-duct intraductal papillary mucinous neoplasms of the pancreas: to operate or not to operate? Gut. 2007. 56:1086–1090.23. Sugiyama M, Izumisato Y, Abe N, Masaki T, Mori T, Atomi Y. Predictive factors for malignancy in intraductal papillary-mucinous tumours of the pancreas. Br J Surg. 2003. 90:1244–1249.24. Serikawa M, Sasaki T, Fujimoto Y, Kuwahara K, Chayama K. Management of intraductal papillary-mucinous neoplasm of the pancreas: treatment strategy based on morphologic classification. J Clin Gastroenterol. 2006. 40:856–862.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Visible Intraductal Papillary Mucinous Neoplasm after Drinking Water
- Intraductal Papillary Mucinous Tumor Simultaneously Involving the Liver and Pancreas: A Case Report
- Surgical Management for Intraductal Papillary Mucinous Tumor (IPMT) of the Pancreas Confined to Branch Duct
- Determination of Malignancy Predictors in Branch Duct TypeIntraductal Papillary Mucinous Neoplasms of the Pancreas
- Pathologic View of Intraductal Papillary Mucinous Neoplasm