J Korean Med Sci.
2007 Dec;22(6):1026-1033. 10.3346/jkms.2007.22.6.1026.
Inflammatory and Remodeling Events in Asthma with Chronic Exposure to House Dust Mites: A Murine Model
- Affiliations
-
- 1Department of Internal Medicine, The Catholic University of Korea, College of Medicine, Seoul, Korea. sskwon@catholic.ac.kr
- KMID: 1785765
- DOI: http://doi.org/10.3346/jkms.2007.22.6.1026
Abstract
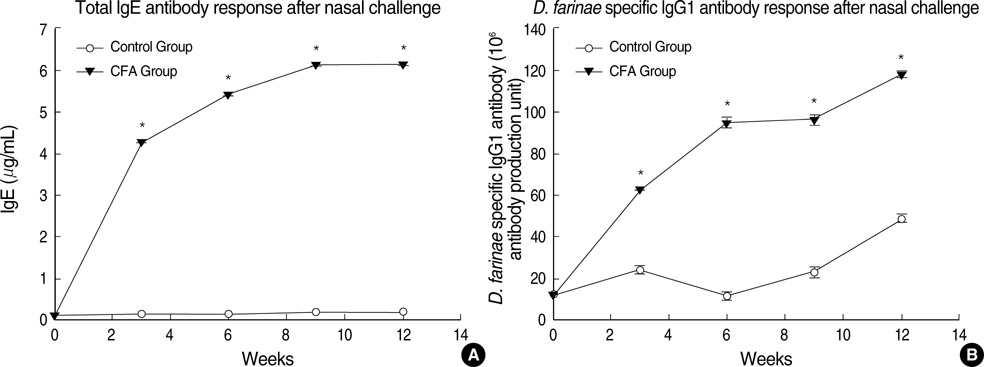
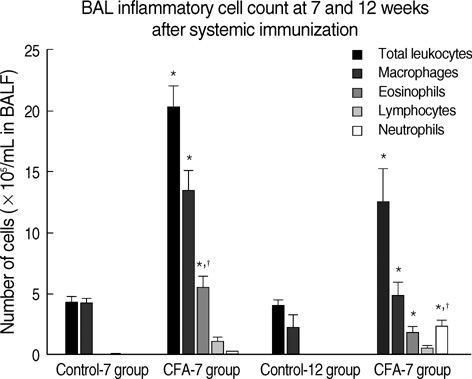
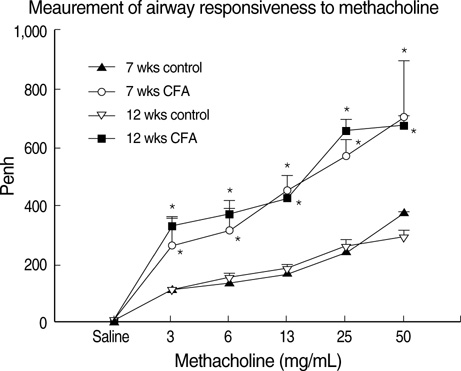
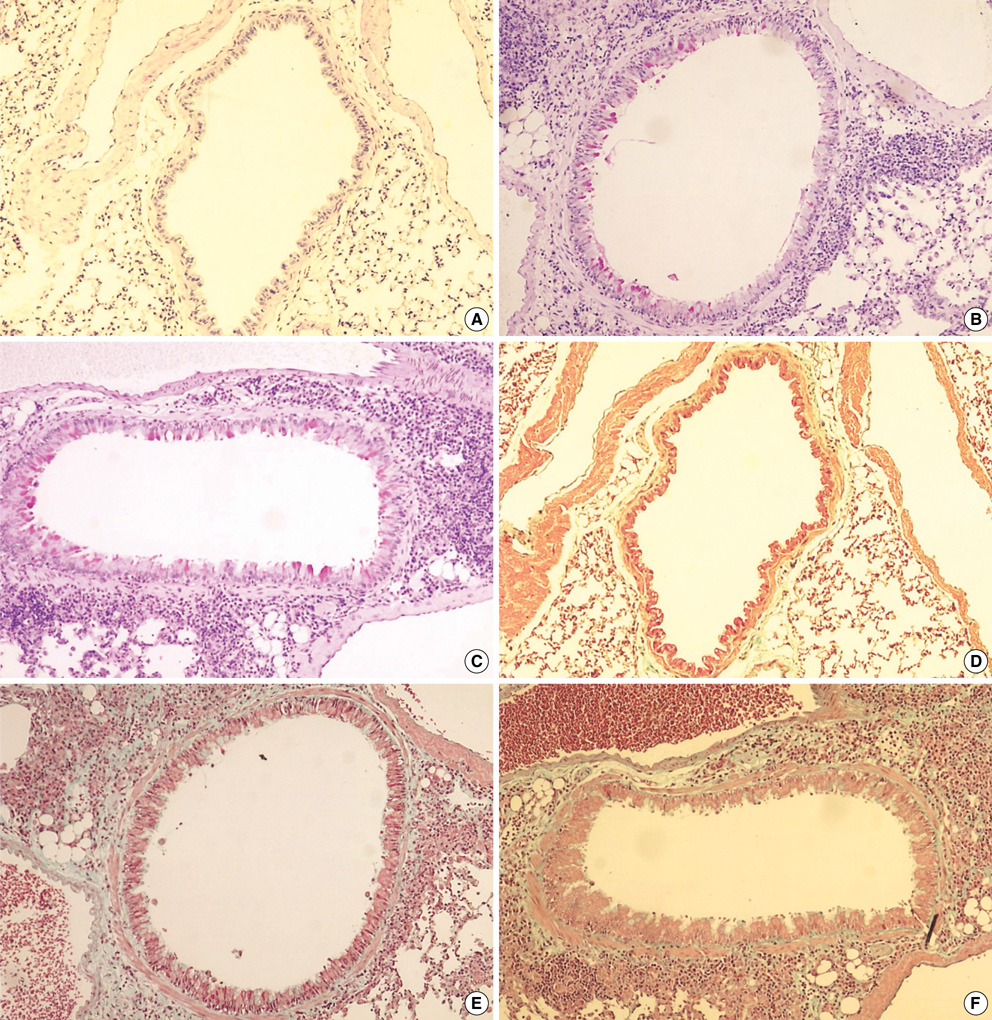
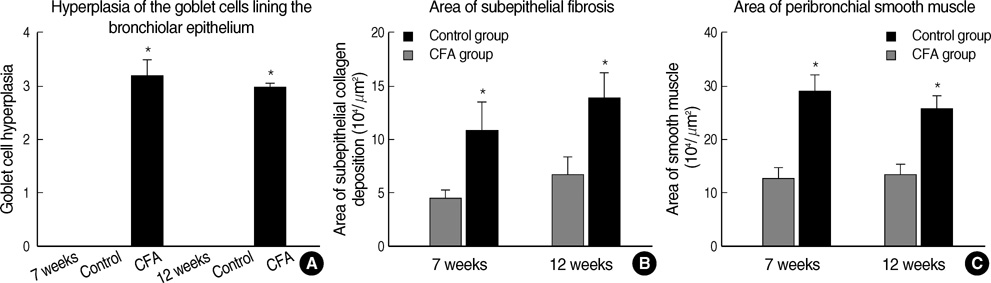
- Although animal models with ovalbumin have been used to study chronic asthma, there are difficulties in inducing recurrence as well as in maintaining chronic inflammation in this system. Using a murine model of house dust mite (HDM)-induced bronchial asthma, we examined the airway remodeling process in response to the chronic exposure to HDM. During the seventh and twelfth weeks of study, HDM were inhaled through the nose for three consecutive days and airway responsiveness was measured. Twenty-four hours later, bronchoalveolar lavage and histological examination were performed. The degree of overproduction of mucus, subepithelial fibrosis, and the thickness of the peribronchial smooth muscle in the experimental group was clearly increased compared to the control group. In addition, HDM-exposed mice demonstrated severe airway hyperreactivity to methacholine. In the bronchoalveolar lavage fluid, the number of total cells and eosinophils was increased; during the twelfth week, the number of neutrophils increased in the experimental group. With regard to changes in cytokines, the concentrations of IL-4, IL- 13, and transforming growth factor-beta (TGF-beta) were increased in the experimental group. The data suggest that eosinophils, IL-4, IL-13, and TGF-beta might play an important role in the airway remodeling process and that neutrophils may be involved with increased exposure time.
Keyword
MeSH Terms
Figure
Reference
-
1. Busse WW, Lemanske RF Jr. Asthma. N Engl J Med. 2001. 344:350–362.
Article2. Boulet LP, Turcotte H, Laviolette M, Naud F, Bernier MC, Martel S, Chakir J. Airway hyperresponsiveness, inflammation, and subepithelial collagen deposition in recently diagnosed versus long-standing mild asthma: influence of inhaled corticosteroids. Am J Respir Crit Care Med. 2000. 162:1308–1313.3. Laprise C, Laviolette M, Boutet M, Boulet LP. Asymptomatic airway hyperresponsiveness: relationships with airway inflammation and remodeling. Eur Respir J. 1999. 14:63–73.4. Fish JE, Peters SP. Airway remodeling and persistent airway obstruction in asthma. J Allergy Clin Immunol. 1999. 104:509–516.
Article5. Kips JC, Pauwels RA. Airway wall remodelling: does it occur and what does it mean? Clin Exp Allergy. 1999. 29:1457–1466.
Article6. Davies DE, Wicks J, Powell RM, Puddicombe SM, Holgate ST. Airway remodeling in asthma: New insights. J Allergy Clin Immunol. 2003. 111:215–225.7. Macklem PT. A theoretical analysis of the effect of airway smooth muscle load on airway narrowing. Am J Respir Crit Care Med. 1996. 153:83–89.
Article8. Solway J, Fredberg JJ. Perhaps airway smooth muscle dysfunction contributes to asthmatic bronchial hyperresponsiveness after all. Am J Respir Cell Mol Biol. 1997. 17:144–146.
Article9. Wenzel SE, Szefler SJ, Leung DY, Sloan SI, Rex MD, Martin RJ. Bronchoscopic evaluation of severe asthma. Persistent inflammation associated with high dose glucocorticoids. Am J Respir Crit Care Med. 1997. 156:737–743.10. Cox G. The role of neutrophils in inflammation. Can Respir J. 1998. 5:Suppl A. 37A–40A.11. Wenzel SE. The significance of the neutrophil in asthma. Clin Exp All Rev. 2001. 1:89–92.
Article12. Swirski FK, Sajic D, Robbins CS, Gajewska BU, Jordana M, Stampfli MR. Chronic exposure to innocuous antigen in sensitized mice leads to suppressed airway eosinophilia that is reversed by granulocyte macrophage colony-stimulating factor. J Immunol. 2002. 169:3499–3506.
Article13. Kim JS, Lee JM, Kim SJ, Lee SY, Kwon SS, Kim YK, Kim KH, Moon HS, Song JS, Park SH. The preventive effect by induction of oral tolerance in a mouse model of allergic asthma. Tuberc Respir Dis. 2004. 57:425–433.14. Johnson JR, Wiley RE, Fattouh R, Swirski FK, Gajewska BU, Coyle AJ, Gutierrez-Ramos JC, Ellis R, Inman MD, Jordana M. Continuous exposure to house dust mite elicits chronic airway inflammation and structural remodeling. Am J Respir Crit Care Med. 2004. 169:378–385.
Article15. Wolfowicz CB, HuangFu T, Chua KY. Expression and immunogenicity of the major house dust mite allergen Der p 1 following DNA immunization. Vaccine. 2003. 21:1195–1204.
Article16. Tanaka H, Masuda T, Tokuoka S, Komai M, Nagao K, Takahashi Y, Nagai H. The effect of allergen-induced airway inflammation on airway remodeling in a murine model of allergic asthma. Inflamm Res. 2001. 50:616–624.
Article17. Ellis R, Leigh R, Southam D, O'Byrne PM, Inman MD. Morphometric analysis of mouse airways after chronic allergen challenge. Lab Invest. 2003. 83:1285–1291.
Article18. Leigh R, Ellis R, Wattie J, Southam DS, De Hoogh M, Gauldie J, O'Byrne PM, Inman MD. Dysfunction and remodeling of the mouse airway persist after resolution of acute allergen induced inflammation. Am J Respir Cell Mol Biol. 2002. 27:526–535.19. Fahy JV. Remodeling of the airway epithelium in asthma. Am J Respir Crit Care Med. 2001. 164:S46–S51.
Article20. Djukanovic R. Airway inflammation in asthma and its consequences: Implications for treatment in children and adults. J Allergy Clin Immunol. 2002. 109:S539–S548.
Article21. Shinagawa K, Kojima M. Mouse model of airway remodeling: strain differences. Am J Respir Crit Care Med. 2003. 168:959–967.22. Kim SJ, Lee SY, Park YK, Kim MH, Kim SC, Kwon SS, Kim YK, Kim KH, Moon HS, Song JS, Park SH. The relationship between airway remodeling and transforming growth factor-β 1 and basic fibroblast growth factor expression in bronchoalveolar cells from asthmatics. J Asthma Allergy Clin Immunol. 2002. 22:532–539.23. Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active Transforming Growth Factor-β 1 induces prolonged severe fibrosis in rat lung. J Clin Invest. 1997. 100:768–776.24. Chu HW, Trudeau JB, Balzar S, Wenzel SE. Peripheral blood and airway tissue expression of transforming growth factor β by neutrophils in asthmatic subjects and normal control subjects. J Allergy Clin Immunol. 2000. 106:1115–1123.25. Takeyama K, Dabbagh K, Jeong Shim J, Dao-Pick T, Ueki IF, Nadel JA. Oxidative stress causes mucin synthesis via transactivation of epidermal growth factor receptor: role of neutrophils. J Immunol. 2000. 164:1546–1552.
Article26. Taube C, Dakhama A, Takeda K, Nick JA, Gelfand EW. Allergen-specific early neutrophil infiltration after allergen challenge in a murine model. Chest. 2003. 123(Supple 3):410S–411S.
Article27. Corbel M, Caulet-Maugendre S, Germain N, Lagente V, Boichot E. Enhancement of gelatinase activity during development of subepithelial fibrosis in a murine model of asthma. Clin Exp Allergy. 2003. 33:696–704.
Article28. Billiau A, Matthys P. Modes of action of Freund's adjuvants in experimental models of autoimmune diseases. J Leukoc Biol. 2001. 70:849–860.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Establishment of a murine model based on chronic exposure to domestic house dust mites in Korea
- An Ecological Study on the House Dust Mite
- Repellent effect of Mate tea and Jasmine tea against house dust mites (Dermatophagoides farinae and D. pteronyssinus)
- Distribution of House Dust Mites in the Bedroom of Patients with Allergic Rhinitis in Pusan Area
- Sensitization of house dust mites in the allergic patients and mite ecology in their house dusts