J Korean Med Sci.
2004 Feb;19(1):107-112. 10.3346/jkms.2004.19.1.107.
Bystander-Mediated Regression of Murine Neuroblastoma via Retroviral Transfer of the HSV-TK Gene
- Affiliations
-
- 1Department of Pediatrics, Hallym University College of Medicine, Seoul, Korea. mkkim@sunlin.com
- 2Department of Pediatrics, Handong University Good Samaritan Hospital, Pohang, Korea.
- KMID: 1785703
- DOI: http://doi.org/10.3346/jkms.2004.19.1.107
Abstract
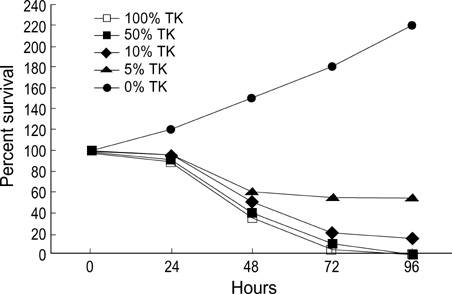
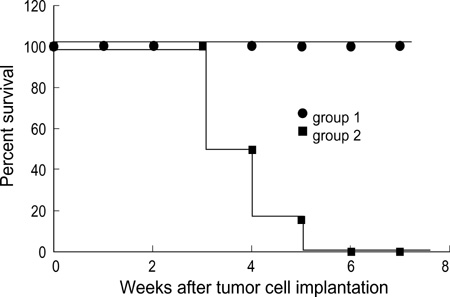
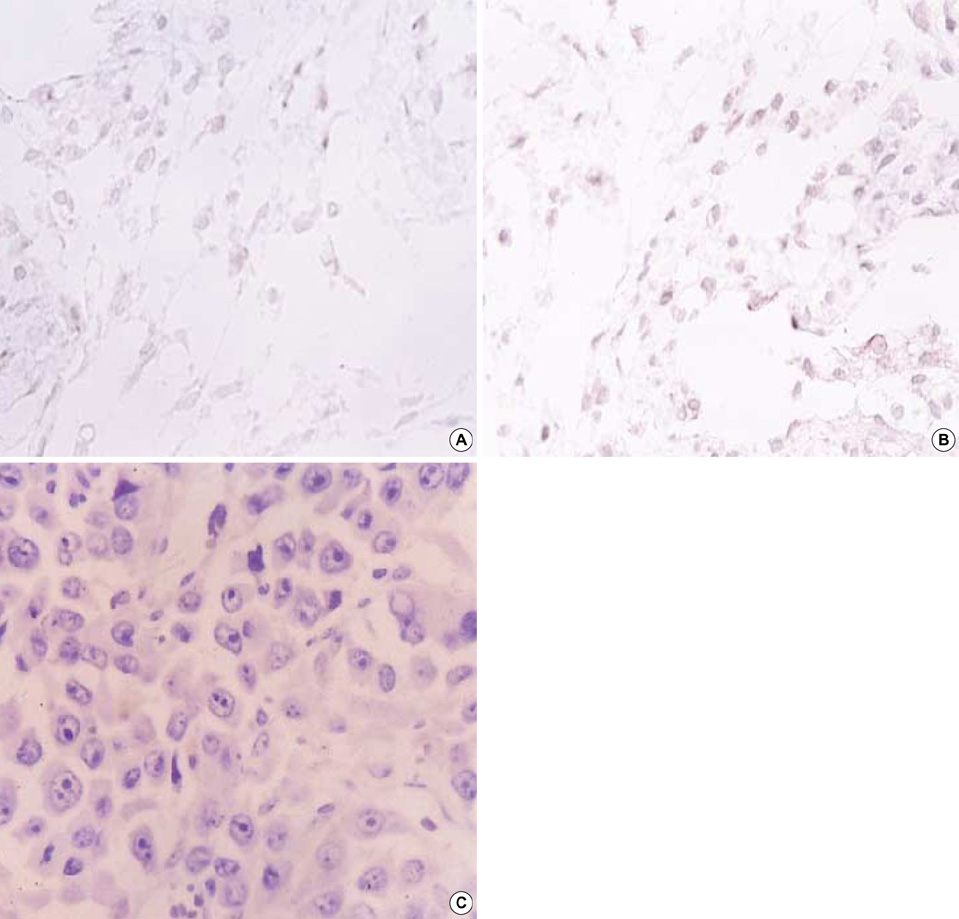
- Selective introduction of genes conferring chemosensitivity into proliferating tumor cells may be used to treat cancer. We investigated the bystander effect of retrovirusmediated gene transfer of herpes simplex virus thymidine kinase (HSV-TK) gene to murine neuroblastoma cell line (neuro-2a) in vitro and in vivo, and we examined whether the mechanism of bystander effect in neuroblastoma would also depend on connexin-dependent gap junction and/or immune response. A strong bystander effect was observed in vitro, whereby nontransduced tumor cells in proximity to transduced cells acquired susceptibility to ganciclovir (GCV) killing. Implanted mixtures of wildtype cells and HSV-TK transduced cells showed a potent bystander effect upon administration of GCV in A/J mice. HSV-TK/GCV system in murine neuroblastoma induced systemic immunity. Immunohistochemical staining showed many CD4+ and CD8+ cell infiltration but did not show anti-connexin 43+ cells. In conclusion, a strong bystander effect was observed in vitro and in vivo. The bystander effect in murine neuroblastoma might be dependent on immune response and/or on other mechanism such as protein phosphorylation or transfer of apoptotic vesicle, rather than connexin-dependent gap junction.
Keyword
MeSH Terms
-
Animals
Apoptosis
Bystander Effect
CD4-Positive T-Lymphocytes/metabolism
CD8-Positive T-Lymphocytes/metabolism
Cell Line, Tumor
Connexin 43/biosynthesis
Gap Junctions
Gene Therapy/*methods
*Gene Transfer Techniques
Human
Immunohistochemistry
Mice
Neoplasm Transplantation
Neuroblastoma/*therapy
Phosphorylation
Retroviridae/genetics
Simplexvirus/*enzymology
Support, Non-U.S. Gov't
Thymidine Kinase/*genetics
Time Factors
Figure
Reference
-
1. Freeman SM, Abboud CN, Whartenby KA, Packman CH, Koeplin DS, Moolten FL, Abraham GN. The bystander effect: tumor regression when a fraction of the tumor mass is genetically modified. Cancer Res. 1993. 53:5274–5283.2. Touraine RL, Ishii-Morita H, Ramsey WJ, Blaese RM. The bystander effect in the HSVtk/ganciclovir system and its relationship to gap junctional communication. Gene Ther. 1998. 5:1705–1711.
Article3. Estin D, Li M, Spray D, Wu JK. Connexins are expressed in primary brain tumors and enhance the bystander effect in gene therapy. Neurosurgery. 1999. 44:361–368.
Article4. Duflot-Dancer A, Piccoli C, Rolland A, Yamasaki H, Mesnil M. Longterm connexin-mediated bystander effect in highly tumorigenic human cells in vivo in herpes simplex virus thymidine kinase/ganciclovir gene therapy. Gene Ther. 1998. 5:1372–1378.
Article5. McMasters RA, Saylors RL, Jones KE, Hendrix ME, Moyer MP, Drake RR. Lack of bystansder killing in herpes simplex virus thymidine kinase-transduced colon cell lines due to deficient connexin 43 gap junction formation. Hum Gene Ther. 1998. 9:2253–2261.6. Colombo BM, Benedetti S, Ottolenghi S, Mora M, Pollo B, Poli G, Finocchiaro G. The bystander effect: Association of U-87 cell death with Ganciclovir-mediated apoptosis of nearby cells and lack of effect in athymic mice. Hum Gene Ther. 1995. 6:763–772.
Article7. Vile RG, Nelson JA, Castleden S, Chong H, Hart IR. Systemic gene therapy of murine melanoma using tissue specific expression of the HSVtk gene involves an immune component. Cancer Res. 1994. 54:6228–6234.8. Caruso M, Panis Y, Gagandeep S, Houssin D, Salzmann JL, Klatzmann D. Regression of established macroscopic liver metastases after in situ transduction of a suicide gene. Proc Natl Acad Sci USA. 1993. 90:7024–7028.
Article9. Barba D, Hardin J, Sadelain M, Gage FH. Development of anti-tumor immunity following thymidine kinase-mediated killing of experimental brain tumors. Proc Natl Acad Sci USA. 1994. 91:4348–4352.
Article10. Ramesh R, Munshi A, Abboud CN, Marrogi AJ, Freeman SM. Expression of costimulatory molecules: B7 and ICAM up-regulation after treatment with a suicide gene. Cancer Gene Ther. 1996. 3:373–384.11. Puumalainen AM, Vapalahti M, Agrawal RS, Kossila M, Laukkanen J, Lehtolainen P, Viita H, Paljarvi L, Vanninen R, Yla-Herttuala S. Beta- galactosidase gene transfer to human malignant glioma in vivo using replication-deficient retroviruses and adenoviruses. Hum Gene Ther. 1998. 9:1769–1774.12. Sandmair AM, Turunen M, Tyynela K, Loimas S, Vainio P, Vanninen R, Vapalahti M, Bjerkvig R, Janne J, Yla-Herttuala S. Herpes simplex virus thymidine kinase gene therapy in experimental rat BT4C glioma model: effect of the percentage of thymidine kinase-positive glioma cells on treatment effect, survival time, and tissue reactions. Cancer Gene Ther. 2000. 7:413–421.
Article13. Walling HW, Swarthout JT, Culver KW. Bystander-mediated regression of osteosarcoma via retroviral transfer of the herpes simplex virus thymidine kinase and human interleukin-2 genes. Cancer Gene Ther. 2000. 7:187–196.
Article14. Tapscott SJ, Miller AD, Olson JM, Berger MS, Groudine M, Spence AM. Gene therapy of rat 9L gliosarcoma tumors by transduction with selectable genes does not require drug selection. Proc Natl Acad Sci USA. 1994. 91:8185–8189.
Article15. Barth RF. Rat brain tumor models in experimental neuro-oncology: the 9L, C6, T9, F98, RG2 (D74), RT-2 and CNS-1 gliomas. J Neurooncol. 1998. 36:91–102.16. Ram Z, Walbridge S, Shawker T, Culver KW, Blaese RM, Oldfield EH. The effect of thymidine kinase transduction and ganciclovir therapy on tumor vasculature and growth of 9L gliomas in rats. J Neurosurg. 1994. 81:256–260.
Article17. Ramesh R, Marrogi AJ, Munshi A, Abboud CN, Freeman SM. In vivo analysis of the 'bystander effect': a cytokine cascade. Exp Hematol. 1996. 24:829–838.18. Naus CC, Bechberger JF, Zhang Y, Venance L, Yamasaki H, Juneja SC, Kidder GM, Giaume C. Altered gap junctional communication, intercellular signaling, and growth in cultured astrocytes deficient in connexin 43. J Neurosci Res. 1997. 49:528–540.19. Marconi P, Tamura M, Moriuchi S, Krisky DM, Niranjan A, Goins WF, Cohen JB, Glorioso JC. Connexin 43-enhanced suicide gene therapy using herpesviral vectors. Mol Ther. 2000. 1:71–81.
Article20. Samejima Y, Meruelo D. 'Bystander killing' induces apoptosis and is inhibited by forskolin. Gene Ther. 1995. 2:50–58.21. Hamel W, Magnelli L, Chiarugi VP, Israel MA. Herpes simplex virus thymidine kinase/ganciclovir-mediated apoptotic death of bystander cells. Cancer Res. 1996. 56:2697–2702.22. Dahle J, Mikalsen SO, Rivedal E, Steen HB. Gap junctional intercellular communication is not a major mediator in the bystander effect in photodynamic treatment of MDCK II cells. Radiat Res. 2000. 154:331–341.
Article23. Andrade-Rozental AF, Rozental R, Hopperstad MG, Wu JK, Vrionis FD, Spray DC. Gap junctions: the "kiss of death" and the "kiss of life". Brain Res Rev. 2000. 308–315.
Article24. Agard C, Ligeza C, Dupas B, Izembart A, El Kouri C, Moullier P, Ferry N. Immune-dependent distant bystander effect after adenovirus-mediated suicide gene transfer in a rat model of liver colorectal metastasis. Cancer Gene Ther. 2001. 8:128–136.
Article25. Nagy HJ, Panis Y, Fabre M, Engelmann C, Soubrane O, Houssin D, Klatzmann D. Efficient suicide gene therapy of transduced and distant untransduced ovary tumors is correlated with significant increase of intratumoral T and NK cells. Biomed Pharmacother. 2000. 54:479–486.
Article26. Cho HS, Song JY, Park CY, Lyu CJ, Kim BS, Kim KY. Retroviral-mediated IL-2 gene transfer into murine neuroblastoma. Yonsei Med J. 2000. 41:76–81.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Study of the Bystander Effect and Its Enhancement in HSV-TK Gene Therapy Using a Murine Neuroblastoma Model
- Bystander Effect of HSV-TK/GCV Gene Therapy in Murine Neuroblastoma
- Herpes Simplex virus thymidine kinase gene therapy delivered by retroviral or adenoviral vector in mouse model of lewis lung carcinoma
- Effect of GCV on Neuroblastoma Cell Line Expressed by HSV-TK Gene with Retroviral Vector
- Gene Transfer Effects of Thymidine Kinase Gene of Herpes Simplex Type 1 on Ganciclovir Cytotoxicity in Gastric Cancer Cell Line