J Korean Med Assoc.
2013 Dec;56(12):1076-1083. 10.5124/jkma.2013.56.12.1076.
Antidotes of cyanide intoxication
- Affiliations
-
- 1Department of Emergency Medicine, Korea University College of Medicine, Seoul, Korea.
- 2Department of Emergency Medicine, Inha University College of Medicine, Incheon, Korea. jskimmd@inha.ac.kr
- KMID: 1783722
- DOI: http://doi.org/10.5124/jkma.2013.56.12.1076
Abstract
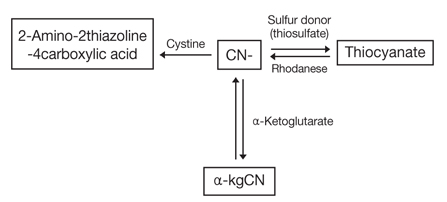
- Cyanide poisoning can occur from industrial disasters, smoke inhalation from fire, food, and multiple other sources. Cyanide inhibits mitochondrial oxidative phosphorylation by blocking mitochondrial cytochrome oxidase, which in turn results in anaerobic metabolism and depletion of adenosine triphosphate in cells. Rapid administration of antidote is crucial for life saving in severe cyanide poisoning. Multiple antidotes are available for cyanide poisoning. The action mechanism of cyanide antidotes include formation of methemoglobin, production of less or no toxic complex, and sulfane sulfur supplementation. At present, the available antidotes are amyl nitrite, sodium nitrite, sodium thiosulfate, hydroxocobalamin, 4-dimethylaminophenol, and dicobalt edetate. Amyl nitrite, sodium nitrite, and 4-dimethylaminophenol induce the formation of methemoglobin. Sodium thiosulfate supplies the sulfane sulfur molecule to rhodanese, allowing formation of thiocyanate and regeneration of native enzymes. Hydroxocobalamin binds cyanide rapidly and irreversibly to form cyanocobalamin. Dicobalt edetate acts as a chelator of cyanide, forming a stable complex. Based on the best evidence available, a treatment regimen of 100% oxygen and hydroxocobalamin, with or without sodium thiosulfate, is recommended for cyanide poisoning. Amyl nitrite and sodium nitrite, which induce methemoglobin, should be avoided in victims of smoke inhalation because of serious adverse effects.
MeSH Terms
-
Adenosine Triphosphate
Aminophenols
Amyl Nitrite
Antidotes*
Disasters
Edetic Acid
Electron Transport Complex IV
Equipment and Supplies
Fires
Hydroxocobalamin
Inhalation
Metabolism
Methemoglobin
Oxidative Phosphorylation
Oxygen
Poisoning
Polyphosphates
Regeneration
Smoke
Sodium
Sodium Nitrite
Sulfur
Thiocyanates
Thiosulfate Sulfurtransferase
Thiosulfates
Vitamin B 12
Adenosine Triphosphate
Aminophenols
Amyl Nitrite
Antidotes
Edetic Acid
Electron Transport Complex IV
Hydroxocobalamin
Methemoglobin
Oxygen
Polyphosphates
Smoke
Sodium
Sodium Nitrite
Sulfur
Thiocyanates
Thiosulfate Sulfurtransferase
Thiosulfates
Vitamin B 12
Figure
Cited by 1 articles
-
Management of Cyanide Intoxication with Extracorporeal Membrane Oxygenation and Continuous Renal Replacement Therapy
Jin Park, Seung-Yeob Lee, Hyun-Sik Choi, Yoon Hee Choi, Young-Joo Lee
Korean J Crit Care Med. 2015;30(3):218-221. doi: 10.4266/kjccm.2015.30.3.218.
Reference
-
1. Holstege CP, Isom GE, Kirk MA. Cyanide and hydrogen sulfide. In : Nelson LS, Goldfrank LR, editors. Goldfrank's toxicologic emergencies. 9th ed. NewYork: McGraw-Hill Medical;2011. p. 1712–1724.2. National Fire Data System. 2010 annual report for fire statistics [Internet]. Seoul: National Fire Data System;2010. cited 2013 Aug 18. Available from: http://www.nfds.go.kr/ebook/2010/all/ebook01.html.3. Sauer SW, Keim ME. Hydroxocobalamin: improved public health readiness for cyanide disasters. Ann Emerg Med. 2001; 37:635–641.
Article4. Fortin JL, Giocanti JP, Ruttimann M, Kowalski JJ. Prehospital administration of hydroxocobalamin for smoke inhalation-associated cyanide poisoning: 8 years of experience in the Paris Fire Brigade. Clin Toxicol (Phila). 2006; 44:Suppl 1. 37–44.
Article5. Guidotti T. Acute cyanide poisoning in prehospital care: new challenges, new tools for intervention. Prehosp Disaster Med. 2006; 21:s40–s48.
Article6. Borron SW, Baud FJ. Antidotes for acute cyanide poisoning. Curr Pharm Biotechnol. 2012; 13:1940–1948.
Article7. Sather JE, Tantawy H. Toxins. Anesthesiol Clin. 2006; 24:647–670.
Article8. Mitchell BL, Bhandari RK, Bebarta VS, Rockwood GA, Boss GR, Logue BA. Toxicokinetic profiles of α-ketoglutarate cyanohydrin, a cyanide detoxification product, following exposure to potassium cyanide. Toxicol Lett. 2013; 222:83–89.
Article9. Baud FJ. Cyanide: critical issues in diagnosis and treatment. Hum Exp Toxicol. 2007; 26:191–201.
Article10. Ellenhorn MJ, Barceloux DG. Ellenhorn's medical toxicology. 2nd ed. Baltimore: Williams & Wilkins;1997.11. Baud FJ, Borron SW, Megarbane B, Trout H, Lapostolle F, Vicaut E, Debray M, Bismuth C. Value of lactic acidosis in the assessment of the severity of acute cyanide poisoning. Crit Care Med. 2002; 30:2044–2050.
Article12. Baud FJ, Barriot P, Toffis V, Riou B, Vicaut E, Lecarpentier Y, Bourdon R, Astier A, Bismuth C. Elevated blood cyanide concentrations in victims of smoke inhalation. N Engl J Med. 1991; 325:1761–1766.
Article13. Baud FJ, Borron SW, Bavoux E, Astier A, Hoffman JR. Relation between plasma lactate and blood cyanide concentrations in acute cyanide poisoning. BMJ. 1996; 312:26–27.
Article14. Eckstein M, Maniscalco PM. Focus on smoke inhalation: the most common cause of acute cyanide poisoning. Prehosp Disaster Med. 2006; 21:s49–s55.15. Vanden Hoek TL, Morrison LJ, Shuster M, Donnino M, Sinz E, Lavonas EJ, Jeejeebhoy FM, Gabrielli A. Part 12: cardiac arrest in special situations: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010; 122:18 Suppl 3. S829–S861.16. Hung YM, Hung SY, Olson KR, Chou KJ, Lin SL, Chung HM, Tung CN, Chang JC. Yam bean seed poisoning mimicking cyanide intoxication. Intern Med J. 2007; 37:130–132.
Article17. Hall AH, Dart R, Bogdan G. Sodium thiosulfate or hydroxocobalamin for the empiric treatment of cyanide poisoning? Ann Emerg Med. 2007; 49:806–813.
Article18. Klimmek R, Krettek C, Szinicz L, Eyer P, Weger N. Effects and biotransformation of 4-dimethylaminophenol in man and dog. Arch Toxicol. 1983; 53:275–288.
Article19. Kerger H, Dodidou P, Passani-Kruppa D, Gruttner J, Birmelin M, Volz A, Waschke KF. Excessive methaemoglobinaemia and multi-organ failure following 4-DMAP antidote therapy. Resuscitation. 2005; 66:231–235.
Article20. Satpute RM, Hariharakrishnan J, Bhattacharya R. Alpha-ketoglutarate and N-acetyl cysteine protect PC12 cells from cyanide-induced cytotoxicity and altered energy metabolism. Neurotoxicology. 2008; 29:170–178.
Article21. Satpute RM, Hariharakrishnan J, Bhattacharya R. Effect of alpha-ketoglutarate and N-acetyl cysteine on cyanide-induced oxidative stress mediated cell death in PC12 cells. Toxicol Ind Health. 2010; 26:297–308.
Article22. Mittal G, Singh T, Kumar N, Bhatnagar A, Tripathi RP, Tulsawani R, Vijayaraghavan R, Bhattacharya R. Radiolabeling and dose fixation study of oral alpha-ketoglutarate as a cyanide antidote in healthy human volunteers. Clin Toxicol (Phila). 2010; 48:509–515.
Article23. Broderick KE, Potluri P, Zhuang S, Scheffler IE, Sharma VS, Pilz RB, Boss GR. Cyanide detoxification by the cobalamin precursor cobinamide. Exp Biol Med (Maywood). 2006; 231:641–649.
Article24. Broderick KE, Balasubramanian M, Chan A, Potluri P, Feala J, Belke DD, McCulloch A, Sharma VS, Pilz RB, Bigby TD, Boss GR. The cobalamin precursor cobinamide detoxifies nitroprusside-generated cyanide. Exp Biol Med (Maywood). 2007; 232:789–798.25. Chan A, Balasubramanian M, Blackledge W, Mohammad OM, Alvarez L, Boss GR, Bigby TD. Cobinamide is superior to other treatments in a mouse model of cyanide poisoning. Clin Toxicol (Phila). 2010; 48:709–717.
Article26. Niknahad H, Ghelichkhani E. Antagonism of cyanide poisoning by dihydroxyacetone. Toxicol Lett. 2002; 132:95–100.
Article27. Niknahad H, O'Brien PJ. Antidotal effect of dihydroxyacetone against cyanide toxicity in vivo. Toxicol Appl Pharmacol. 1996; 138:186–191.28. Vick JA, Von Bredow JD. Effectiveness of intramuscularly administered cyanide antidotes on methemoglobin formation and survival. J Appl Toxicol. 1996; 16:509–516.
Article29. Bhattacharya R, Jeevaratnam K, Raza SK, Das Gupta S. Protection against cyanide poisoning by the co-administration of sodium nitrite and hydroxylamine in rats. Hum Exp Toxicol. 1993; 12:33–36.
Article30. Brenner M, Kim JG, Lee J, Mahon SB, Lemor D, Ahdout R, Boss GR, Blackledge W, Jann L, Nagasawa HT, Patterson SE. Sulfanegen sodium treatment in a rabbit model of sub-lethal cyanide toxicity. Toxicol Appl Pharmacol. 2010; 248:269–276.
Article31. Zottola MA, Beigel K, Soni SD, Lawrence R. Disulfides as cyanide antidotes: evidence for a new in vivo oxidative pathway for cyanide detoxification. Chem Res Toxicol. 2009; 22:1948–1953.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- CT Brain Scan in Case of Cyanide Intoxication
- Successful treatment by exchange transfusion of a young infant with sodium nitroprusside poisoning
- Effect of Oxygen on the Antidotal Action of Thiosulfate in Cyanide Poisoning
- Need for stocking of emergency antidotes
- Various injury patterns due to combustion (typical but unfamiliar to physicians and easy to miss) in Korea: a case report