J Periodontal Implant Sci.
2011 Oct;41(5):227-233. 10.5051/jpis.2011.41.5.227.
Response of osteoblast-like cells cultured on zirconia to bone morphogenetic protein-2
- Affiliations
-
- 1Department of Periodontology, Seoul National University School of Dentistry, Seoul, Korea. icrhyu@snu.ac.kr
- 2Department of Prosthodontics, Seoul National University School of Dentistry, Seoul, Korea.
- KMID: 1783617
- DOI: http://doi.org/10.5051/jpis.2011.41.5.227
Abstract
- PURPOSE
The aim of this study was to compare osteoblast behavior on zirconia and titanium under conditions cultured with bone morphogenetic protein-2.
METHODS
MC3T3-E1 cells were cultured on sandblasted zirconia and sandblasted/etched titanium discs. At 24 hours after seeding MC3T3-E1, the demineralized bone matrix (DBM) gel alone and the DBM gel with bone morphogenetic protein-2 (BMP-2) were added to the culture medium. The surface topography was examined by confocal laser scanning microscopy. Cellular proliferation was measured at 1, 4, and 7 days after gel loading. Alkaline phosphatase activity was measured at 7 days after gel loading. The mRNA expression of ALPase, bone sialoprotein, type I collagen, runt-related transcription factor 2 (Runx-2), osteocalcin, and osterix were evaluated by real-time polymerase chain reaction at 4 days and 7 days.
RESULTS
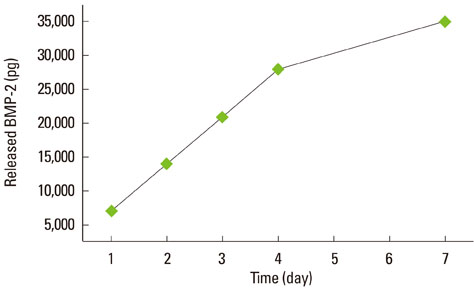
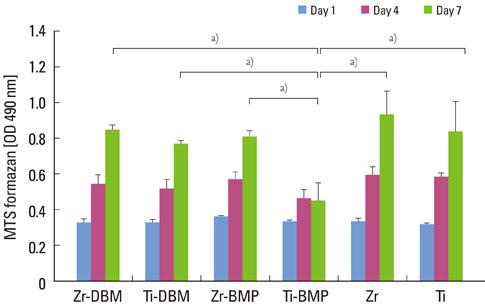
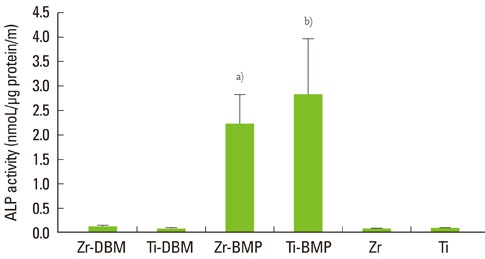
At 1, 4, and 7 days after loading the DBM gel alone and the DBM gel with BMP-2, cellular proliferation on the zirconia and titanium discs was similar and that of the groups cultured with the DBM gel alone and the DBM gel with BMP-2 was not significantly different, except for titanium with BMP-2 gel. ALPase activity was higher in the cells cultured with BMP-2 than in the other groups, but there was no difference between the zirconia and titanium. In ALPase, bone sialoprotein, osteocalcin, Runx-2 and osterix gene expression, that of cells on zirconia or titanium with BMP-2 gel was much more highly increased than titanium without gel at day 7. The gene expression level of cells cultured on zirconia with BMP-2 was higher than that on titanium with BMP-2 at day 7.
CONCLUSIONS
The data in this study demonstrate that the osteoblastic cell attachment and proliferation of zirconia were comparable to those of titanium. With the stimulation of BMP-2, zirconia has a more pronounced effect on the proliferation and differentiation of the osteoblastic cells compared with titanium.
MeSH Terms
-
Alkaline Phosphatase
Bone Matrix
Cell Differentiation
Cell Proliferation
Collagen Type I
Gene Expression
Integrin-Binding Sialoprotein
Microscopy, Confocal
Osteoblasts
Osteocalcin
Real-Time Polymerase Chain Reaction
RNA, Messenger
Seeds
Titanium
Transcription Factors
Zirconium
Alkaline Phosphatase
Collagen Type I
Integrin-Binding Sialoprotein
Osteocalcin
RNA, Messenger
Titanium
Transcription Factors
Zirconium
Figure
Reference
-
1. Schliephake H, Reiss G, Urban R, Neukam FW, Guckel S. Metal release from titanium fixtures during placement in the mandible: an experimental study. Int J Oral Maxillofac Implants. 1993; 8:502–511.2. Urban RM, Jacobs JJ, Tomlinson MJ, Gavrilovic J, Black J, Peoc'h M. Dissemination of wear particles to the liver, spleen, and abdominal lymph nodes of patients with hip or knee replacement. J Bone Joint Surg Am. 2000; 82:457–476.
Article3. Lalor PA, Revell PA, Gray AB, Wright S, Railton GT, Freeman MA. Sensitivity to titanium. A cause of implant failure? J Bone Joint Surg Br. 1991; 73:25–28.
Article4. Valentine-Thon E, Schiwara HW. Validity of MELISA for metal sensitivity testing. Neuro Endocrinol Lett. 2003; 24:57–64.5. Chevalier J. What future for zirconia as a biomaterial? Biomaterials. 2006; 27:535–543.
Article6. Piconi C, Maccauro G. Zirconia as a ceramic biomaterial. Biomaterials. 1999; 20:1–25.
Article7. Akagawa Y, Hosokawa R, Sato Y, Kamayama K. Comparison between freestanding and tooth-connected partially stabilized zirconia implants after two years' function in monkeys: a clinical and histologic study. J Prosthet Dent. 1998; 80:551–558.
Article8. Scarano A, Di Carlo F, Quaranta M, Piattelli A. Bone response to zirconia ceramic implants: an experimental study in rabbits. J Oral Implantol. 2003; 29:8–12.
Article9. Kohal RJ, Weng D, Bächle M, Strub JR. Loaded custom-made zirconia and titanium implants show similar osseointegration: an animal experiment. J Periodontol. 2004; 75:1262–1268.
Article10. Depprich R, Ommerborn M, Zipprich H, Naujoks C, Handschel J, Wiesmann HP, et al. Behavior of osteoblastic cells cultured on titanium and structured zirconia surfaces. Head Face Med. 2008; 4:29.
Article11. Yamashita D, Machigashira M, Miyamoto M, Takeuchi H, Noguchi K, Izumi Y, et al. Effect of surface roughness on initial responses of osteoblast-like cells on two types of zirconia. Dent Mater J. 2009; 28:461–470.
Article12. Kohal RJ, Baechle M, Han JS, Hueren D, Huebner U, Butz F. In vitro reaction of human osteoblasts on alumina-toughened zirconia. Clin Oral Implants Res. 2009; 20:1265–1271.13. Hempel U, Hefti T, Kalbacova M, Wolf-Brandstetter C, Dieter P, Schlottig F. Response of osteoblast-like SAOS-2 cells to zirconia ceramics with different surface topographies. Clin Oral Implants Res. 2010; 21:174–181.
Article14. Suzuki A, Guicheux J, Palmer G, Miura Y, Oiso Y, Bonjour JP, et al. Evidence for a role of p38 MAP kinase in expression of alkaline phosphatase during osteoblastic cell differentiation. Bone. 2002; 30:91–98.
Article15. Sudo H, Kodama HA, Amagai Y, Yamamoto S, Kasai S. In vitro differentiation and calcification in a new clonal osteogenic cell line derived from newborn mouse calvaria. J Cell Biol. 1983; 96:191–198.
Article16. Albrektsson T, Wennerberg A. Oral implant surfaces: Part 1--review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int J Prosthodont. 2004; 17:536–543.17. Albrektsson T, Wennerberg A. Oral implant surfaces: Part 2--review focusing on clinical knowledge of different surfaces. Int J Prosthodont. 2004; 17:544–564.18. He J, Zhou W, Zhou X, Zhong X, Zhang X, Wan P, et al. The anatase phase of nanotopography titania plays an important role on osteoblast cell morphology and proliferation. J Mater Sci Mater Med. 2008; 19:3465–3472.
Article19. Bächle M, Butz F, Hübner U, Bakalinis E, Kohal RJ. Behavior of CAL72 osteoblast-like cells cultured on zirconia ceramics with different surface topographies. Clin Oral Implants Res. 2007; 18:53–59.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Interaction of TGF-beta1 and rhBMP-2 on Human Bone Marrow Stromal Cells Cultured in Collagen Gel Matrix
- Purification of porcine bone morphogenetic protein
- Effect of BMP-7 on the rat periodontal ligament cell
- Fucoidan promotes osteoblast differentiation via JNK- and ERK-dependent BMP2-Smad 1/5/8 signaling in human mesenchymal stem cells
- Response of Human Renal Cell Carcinoma Cells to Bone Morphogenetic Proteins, and the Expression of Bone Morphogenetic Protein Receptors