J Korean Med Sci.
2009 Dec;24(6):1038-1044. 10.3346/jkms.2009.24.6.1038.
Novel CLCN1 Mutations and Clinical Features of Korean Patients with Myotonia Congenita
- Affiliations
-
- 1Department of Neurology, Dae-Dong Hospital, Busan, Korea.
- 2Department of Neurology, Pusan National University School of Medicine, Yangsan, Korea. dskim@pusan.ac.kr
- 3Department of Rehabilitation Medicine, Pusan National University School of Medicine, Yangsan, Korea.
- 4Medical Research Institute, Pusan National University School of Medicine, Yangsan, Korea.
- 5Department of Neurology, College of Medicine, Inje University, Busan, Korea.
- 6Department of Neurology, College of Medicine, Yonsei University, Seoul, Korea.
- KMID: 1783130
- DOI: http://doi.org/10.3346/jkms.2009.24.6.1038
Abstract
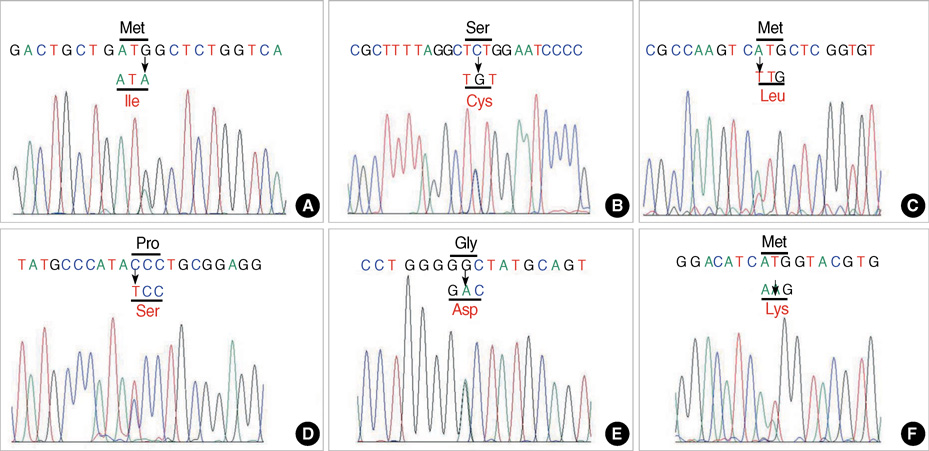
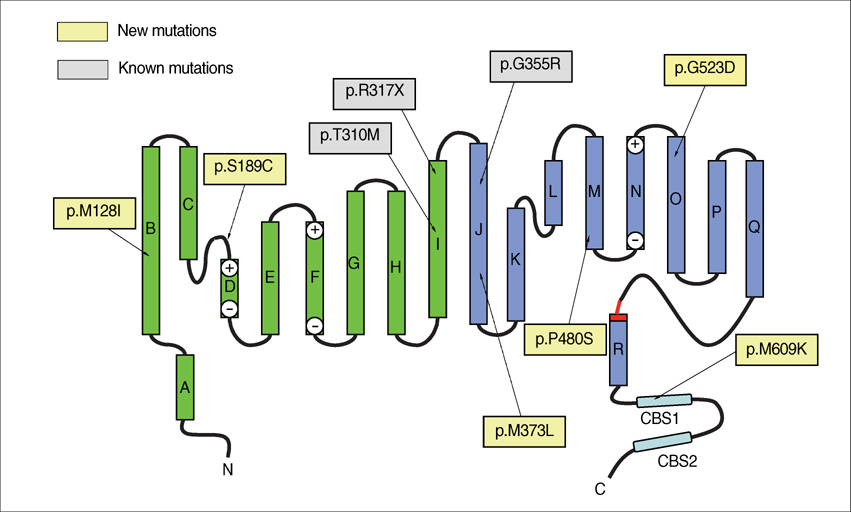
- Myotonia congenita (MC) is a form of nondystrophic myotonia caused by a mutation of CLCN1, which encodes human skeletal muscle chloride channel (CLC-1). We performed sequence analysis of all coding regions of CLCN1 in patients clinically diagnosed with MC, and identified 10 unrelated Korean patients harboring mutations. Detailed clinical analysis was performed in these patients to identify their clinical characteristics in relation to their genotypes. The CLCN1 mutational analyses revealed nine different point mutations. Of these, six (p.M128I, p.S189C, p.M373L, p.P480S, p.G523D, and p.M609K) were novel and could be unique among Koreans. While some features including predominant lower extremity involvement and normal to slightly elevated creatine kinase levels were consistently observed, general clinical features were highly variable in terms of age of onset, clinical severity, aggravating factors, and response to treatment. Our study is the first systematic study of MC in Korea, and shows its expanding clinical and genetic spectrums.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
The Overlap between Fibromyalgia Syndrome and Myotonia Congenita
Tai-Seung Nam, Seok-Yong Choi, Dong-Jin Park, Shin-Seok Lee, Young-Ok Kim, Myeong-Kyu Kim
J Clin Neurol. 2015;11(2):188-191. doi: 10.3988/jcn.2015.11.2.188.Clinical Diversity of SCN4A-Mutation-Associated Skeletal Muscle Sodium Channelopathy
Sang-Chan Lee, Hyang-Sook Kim, Yeong-Eun Park, Young-Chul Choi, Kyu-Hyun Park, Dae-Seong Kim
J Clin Neurol. 2009;5(4):186-191. doi: 10.3988/jcn.2009.5.4.186.
Reference
-
1. Koch MC, Steinmeyer K, Lorenz C, Ricker K, Wolf F, Otto M, Zoll B, Lehmann-Horn F, Grzeschik KH, Jentsch TJ. The skeletal muscle chloride channel in dominant and recessive human myotonia. Science. 1992. 257:797–800.
Article2. George AL Jr, Crackower MA, Abdalla JA, Hudson AJ, Ebers GC. Molecular basis of Thomsen's disease (autosomal dominant myotonia congenita). Nat Genet. 1993. 3:305–310.
Article3. Kubisch C, Schmidt-Rose T, Fontaine B, Bretag AH, Jentsch TJ. ClC-1 chloride channel mutations in myotonia congenita: variable penetrance of mutations shifting the voltage dependence. Hum Mol Genet. 1998. 7:1753–1760.
Article4. Lorenz C, Meyer-Kleine C, Steinmeyer K, Koch MC, Jentsch TJ. Genomic organization of the human muscle chloride channel CIC-1 and analysis of novel mutations leading to Becker-type myotonia. Hum Mol Genet. 1994. 3:941–946.
Article5. Lehmann-Horn F, Rudel R. Molecular pathophysiology of voltage-gated ion channels. Rev Physiol Biochem Pharmacol. 1996. 128:195–268.
Article6. Lossin C, George AL Jr. Myotonia congenita. Adv Genet. 2008. 63:25–55.7. Lehmann-Horn F, Mailander V, Heine R, George AL. Myotonia levior is a chloride channel disorder. Hum Mol Genet. 1995. 4:1397–1402.
Article8. Colding-Jørgensen E, Dunø M, Schwartz M, Vissing J. Decrement of compound muscle action potential is related to mutation type in myotonia congenita. Muscle Nerve. 2003. 27:449–455.
Article9. Dutzler R, Campbell EB, Cadene M, Chait BT, MacKinnon R. X-ray structure of a ClC chloride channel at 3.0 Å reveals the molecular basis of anion selectivity. Nature. 2002. 415:287–294.10. Dutzler R, Campbell EB, MacKinnon R. Gating the selectivity filter in ClC chloride channels. Science. 2003. 300:108–112.
Article11. Fahlke C, Desai RR, Gillani N, George AL Jr. Residues lining the inner pore vestibule of human muscle chloride channels. J Biol Chem. 2001. 276:1759–1765.
Article12. Pusch M. Myotonia caused by mutations in the muscle chloride channel gene CLCN1. Hum Mutat. 2002. 19:423–434.13. Sasaki R, Ichiyasu H, Ito N, Ikeda T, Takano H, Ikeuchi T, Kuzuhara S, Uchino M, Tsuji S, Uyama E. Novel chloride channel gene mutations in two unrelated Japanese families with Becker's autosomal recessive generalized myotonia. Neuromuscul Disord. 1999. 9:587–592.
Article14. Fialho D, Schorge S, Pucovska U, Davies NP, Labrum R, Haworth A, Stanley E, Sud R, Wakeling W, Davis MB, Kullmann DM, Hanna MG. Chloride channel myotonia: exon 8 hot-spot for dominant-negative interactions. Brain. 2007. 130:3265–3274.
Article15. Bateman A. The structure of a domain common to archaebacteria and the homocystinuria disease protein. Trends Biochem Sci. 1997. 22:12–13.
Article16. Scott JW, Hawley SA, Green KA, Anis M, Stewart G, Scullion GA, et al. CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J Clin Invest. 2004. 113:274–284.
Article17. Estévez R, Pusch M, Ferrer-Costa C, Orozco M, Jentsch TJ. Functional and structural conservation of CBS domains from CLC chloride channels. J Physiol. 2004. 557:363–378.
Article18. Deymeer F, Cakirkaya S, Serdaroğlu P, Schleithoff L, Lehmann-Horn F, Rüdel R, Ozdemir C. Transient weakness and compound muscle action potential decrement in myotonia congenita. Muscle Nerve. 1998. 21:1334–1337.
Article19. Meyer-Kleine C, Steinmeyer K, Ricker K, Jentsch TJ, Koch MC. Spectrum of mutations in the major human skeletal muscle chloride channel gene (CLCN1) leading to myotonia. Am J Hum Genet. 1995. 57:1325–1334.20. Wu FF, Ryan A, Devaney J, Warnstedt M, Korade-Mirnics Z, Poser B, Escriva MJ, Pegoraro E, Yee AS, Felice KJ, Giuliani MJ, Mayer RF, Mongini T, Palmucci L, Marino M, Rüdel R, Hoffman EP, Fahlke C. Novel CLCN1 mutations with unique clinical and electrophysiological consequences. Brain. 2002. 125:2392–2407.
Article21. Colding-Jørgensen E. Phenotypic variability in myotonia congenita. Muscle Nerve. 2005. 32:19–34.
Article22. Mailänder V, Heine R, Deymeer F, Lehmann-Horn F. Novel muscle chloride channel mutations and their effects on heterozygous carriers. Am J Hum Genet. 1996. 58:317–324.23. Deymeer F, Lehmann-Horn F, Serdaroğlu P, Çakirkaya S, Benz S, Rüdel R, Ozdemir C. Electrical myotonia in heterozygous carriers of recessive myotonia congenita. Muscle Nerve. 1999. 22:123–125.
Article24. Fialho D, Kullmann DM, Hanna MG, Schorge S. Non-genomic effects of sex hormones on CLC-1 may contribute to gender differences in myotonia congenita. Neuromuscul Disord. 2008. 18:869–872.
Article25. Dunø M, Colding-Jørgensen E, Grunnet M, Jespersen T, Vissing J, Schwartz M. Difference in allelic expression of the CLCN1 gene and the possible influence on the myotonia congenita phenotype. Eur J Hum Genet. 2004. 12:738–743.
Article26. Hakim CA, Thomlinson J. Myotonia congenita in pregnancy. J Obstet Gynaecol Br Commonw. 1969. 76:561–562.
Article27. Michel P, Sternberg D, Jeannet PY, Dunand M, Thonney F, Kress W, Fontaine B, Fournier E, Kuntzer T. Comparative efficacy of repetitive nerve stimulation, exercise, and cold in differentiating myotonic disorders. Muscle Nerve. 2007. 36:643–650.
Article28. Fournier E, Viala K, Gervais H, Sternberg D, Arzel-Hézode M, Laforêf P, Eymard B, Tabti N, Willer JC, Vial C, Fontaine B. Cold extends electromyography distinction between ion channel mutations causing myotonia. Ann Neurol. 2006. 60:356–365.
Article29. Rüdel R, Dengler R, Ricker A, Haass A, Emser W. Improved therapy of myotonia with the lidocaine derivative tocainide. J Neurol. 1980. 222:275–278.
Article30. Meola G, Sansone V. Therapy in myotonic disorders and in muscle channelopathies. Neurol Sci. 2000. 21:5 Suppl. S953–S961.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Phenotypic Difference of CLCN1 Gene Variant (A313T) in a Korean Family with Myotonia Congenita
- Electrophysiological characteristics of R47W and A298T mutations in CLC-1 of myotonia congenita patients and evaluation of clinical features
- Clinical Characteristics and Analysis of CLCN1 in Patients with "EMG Disease"
- Clinical Diversity of SCN4A-Mutation-Associated Skeletal Muscle Sodium Channelopathy
- Myotonia Dystrophica: A Case Report