Infect Chemother.
2012 Feb;44(1):5-10. 10.3947/ic.2012.44.1.5.
Molecular Epidemiologic Analysis of Community-Onset Extended Spectrum Beta-Lactamase (ESBL) Producing Escherichia coli Using Infrequent-Restriction-Site Polymerase Chain Reaction (IRS-PCR) with Comparison by Pulsed-Field Gel Electrophoresis (PFGE)
- Affiliations
-
- 1Department of Biomedical Science, The Catholic University of Korea, Graduate School, Seoul, Korea.
- 2Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea. jhyoo@catholic.ac.kr
- 3Catholic Research Institutes of Medical Science, The Catholic University of Korea, Seoul, Korea.
- KMID: 1782391
- DOI: http://doi.org/10.3947/ic.2012.44.1.5
Abstract
- BACKGROUND
We evaluated the ability of infrequent restriction site-polymerase chain reaction (IRS-PCR) to perform molecular epidemiologic analysis of Community-Onset Extended Spectrum Beta-Lactamase (ESBL) producing Escherichia coli, and also assessed the use of PFGE as an alternative method.
MATERIALS AND METHODS
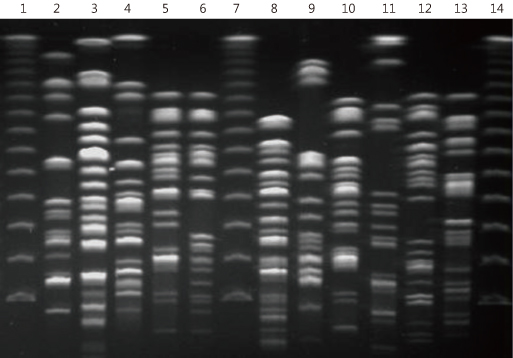
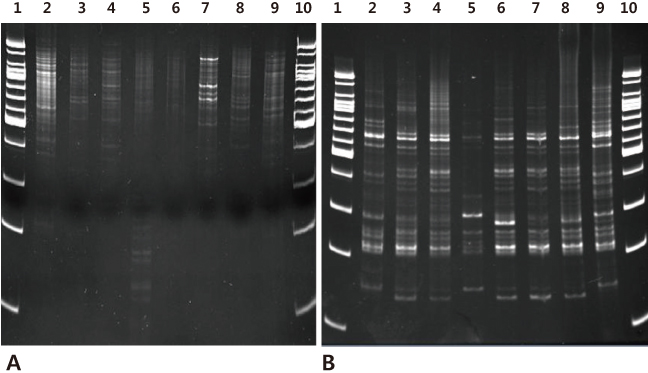
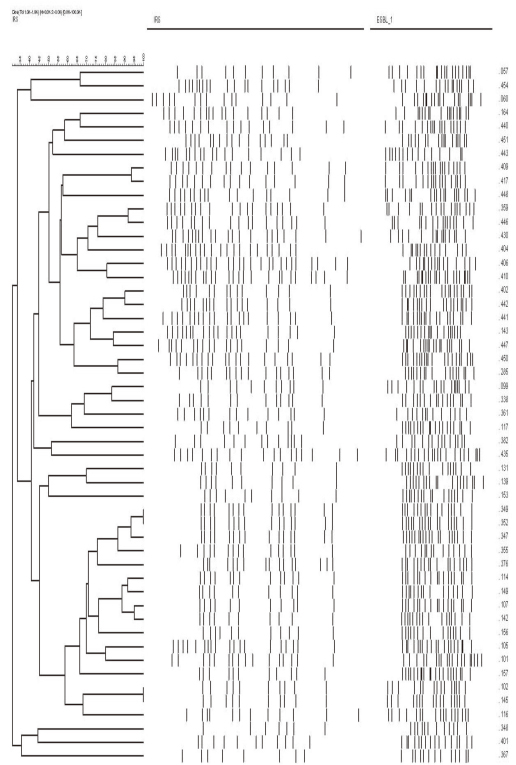
IRS-PCR assay was performed using combinations of adaptors for XbaI and HhaI restriction sites on clinical isolates of E. coli (n=51). We compared the discriminatory power, quality and efficiency of IRS-PCR to PFGE.
RESULTS
In E. coli, PFGE discriminated 39 (76.4%) and IRS-PCR discerned 41 (80.3%) of the total 51 strains. It took much less time to complete IRS-PCR (one day) than PFGE (at least 4 days).
CONCLUSIONS
IRS-PCR is a more sensitive and rapid alternative to PFGE for molecular epidemiologic analysis of E. coli.
Keyword
MeSH Terms
Figure
Reference
-
1. Kim YK, Pai H, Lee HJ, Park SE, Choi EH, Kim J, Kim JH, Kim EC. Bloodstream infections by extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in children: epidemiology and clinical outcome. Antimicrob Agents Chemother. 2002. 46:1481–1491.
Article2. Lee SH, Kim JY, Shin SH, An YJ, Choi YW, Jung YC, Jung HI, Sohn ES, Jeong SH, Lee KJ. Dissemination of SHV-12 and characterization of new AmpC-type beta-lactamase genes among clinical isolates of enterobacter species in Korea. J Clin Microbiol. 2003. 41:2477–2482.
Article3. Pai H, Lee HJ, Choi EH, Kim J, Jacoby GA. Evolution of TEM-related extended-spectrum beta-lactamases in Korea. Antimicrob Agents Chemother. 2001. 45:3651–3653.4. Pai H, Lyu S, Lee JH, Kim J, Kwon Y, Kim JW, Choe KW. Survey of extended-spectrum beta-lactamases in clinical isolates of Escherichia coli and Klebsiella pneumoniae: prevalence of TEM-52 in Korea. J Clin Microbiol. 1999. 37:1758–1763.5. Livermore DM, Canton R, Gniadkowski M, Nordmann P, Rossolini GM, Arlet G, Ayala J, Coque TM, Kern-Zdanowicz I, Luzzaro F, Poirel L, Woodford N. CTX-M: changing the face of ESBLs in Europe. J Antimicrob Chemother. 2007. 59:165–174.
Article6. Rossolini GM, D'Andrea MM, Mugnaioli C. The spread of CTX-M-type extended-spectrum beta-lactamases. Clin Microbiol Infect. 2008. 14:Suppl 1. 33–41.
Article7. Shin WS. Molecular epidemiologic typing Boonja yeokhakjeok hyungbyul. Korean J Infect Dis. 1995. 27:585–594.
Article8. Mazurek GH, Hartman S, Zhang Y, Brown BA, Hector JS, Murphy D, Wallace RJ Jr. Large DNA restriction fragment polymorphism in the Mycobacterium avium-M. intracellulare complex: a potential epidemiologic tool. J Clin Microbiol. 1993. 31:390–394.
Article9. Mazurek GH, Reddy V, Marston BJ, Haas WH, Crawford JT. DNA fingerprinting by infrequent-restriction-site amplification. J Clin Microbiol. 1996. 34:2386–2390.
Article10. Yoo JH. Appbcation of molecular epidemiologic typing to the control of nosocomial infection. Korean J Nosocomial Infect Control. 1997. 2:61–71.
Article11. Bisharat N, Agmon V, Finkelstein R, Raz R, Ben-Dror G, Lerner L, Soboh S, Colodner R, Cameron DN, Wykstra DL, Swerdlow DL, Farmer JJ 3rd. Israel Vibrio Study Group. Clinical, epidemiological, and microbiological features of Vibrio vulnificus biogroup 3 causing outbreaks of wound infection and bacteraemia in Israel. Lancet. 1999. 354:1421–1424.12. Abi-Said D, Anaissie E, Uzun O, Raad I, Pinzcowski H, Vartivarian S. The epidemiology of hematogenous candidiasis caused by different Candida species. Clin Infect Dis. 1997. 24:1122–1128.13. Zhang J, Hollis RJ, Pfaller MA. Variations in DNA subtype and antifungal susceptibility among clinical isolates of Candida tropicalis. Diagn Microbiol Infect Dis. 1997. 27:63–67.
Article14. Kim SI, Yoo JH, Cho YK, Lee DG, Wie SH, Choi JH, Kim YR, Shin WS, Kang MW. Comparison of pulsed-field gel electrophoresis, amplified fragment length polymorphism, and infrequent restriction site-polymerase chain reaction for molecular typing of Escherichia coil and Staphylococcus aureus strains. Korean J Infect Dis. 1999. 31:474–480.
Article15. Yoo JH, Choi JH, Shin WS, Huh DH, Cho YK, Kim KM, Kim MY, Kang MW. Application of infrequent-restriction-site PCR to clinical isolates of Acinetobacter baumannii and Serratia marcescens. J Clin Microbiol. 1999. 37:3108–3112.
Article16. Williams JG, Kubelik AR, Livak KJ, Rafalski JA, Tingey SV. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 1990. 18:6531–6535.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of infrequent restriction site-polymerase chain reaction and pulsed-field gel electrophoresis for molecular typing of Staphylococcus aureus and Escherichia coli
- Comparison of Pulsed-field Gel Electrophoresis, Amplified Fragment Length Polymorphism, and Infrequent Restriction Site-Polymerase Chain Reaction for Molecular Typing of Escherichia coli and Staphylococcus aureus Strains
- In Vitro Susceptibility of piperacillin/tazobactam Against extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae
- Moleculoepidemiological Characteristics of Extended-Spectrum beta-Lactamase Producing Escherichia coli and Klebsiella pneumoniae Strains in Daegu
- Application of Infrequent-Restriction-Site Polymerase Reaction (IRS-PCR) to the Molecular Epidemiologic Analysis of Methicillin Resistant Staphylococcus aureus (MRSA)