J Korean Med Sci.
2005 Oct;20(5):721-726. 10.3346/jkms.2005.20.5.721.
Pharmacokinetics of Glutathione and Its Metabolites in Normal Subjects
- Affiliations
-
- 1Department of Internal Medicine and Clinical Research Institute, Soonchunhyang University Cheonan Hospital, Cheonan, Korea. eylee@sch.ac.kr
- 2Department of Clinical Pharmacology, Soonchunhyang University College of Medicine, Cheonan, Korea.
- 3Proteome Analysis Team, Korea Basic Science Institute, Daejeon, Korea.
- KMID: 1781751
- DOI: http://doi.org/10.3346/jkms.2005.20.5.721
Abstract
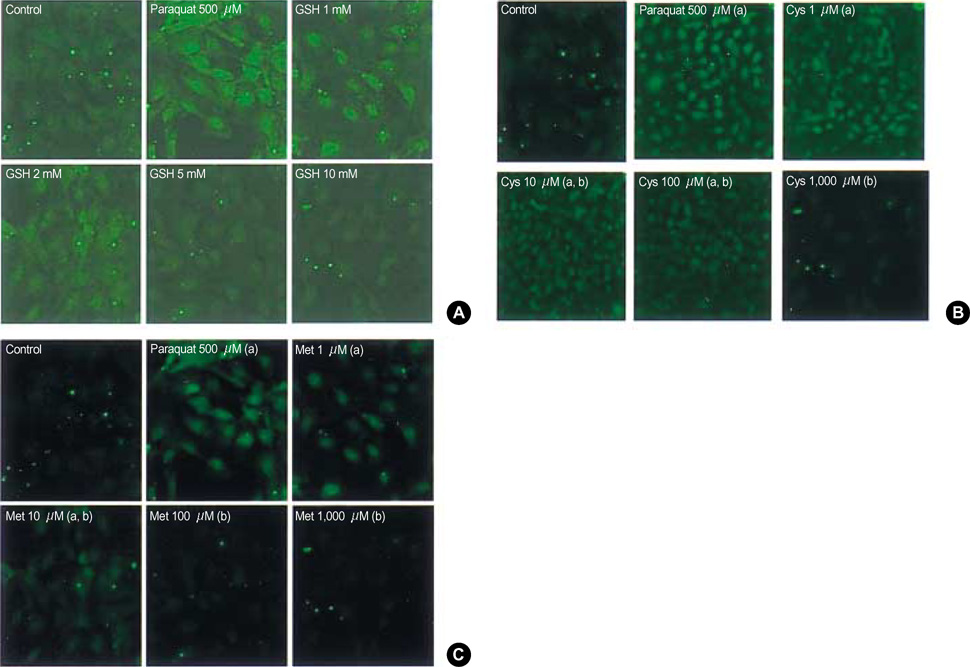
- To determine the loading and maintenance dosage of glutathione (GSH) for patients suffering from reactive oxygen species (ROS) injury such as acute paraquat intoxication, a kinetic study of reduced GSH was performed in synchrony with that of cysteine (Cys), cystine (Cys2), and methionine (Met). Human subject's porticipitation was voluntary. The effective dose of Cys, Cys2, and Met against ROS in fibroblast cells generated by paraquat was assessed using laser scanning confocal microscopy. Both Cys and Met suppressed ROS in a dose-dependent manner at concentrations of 1-1,000 micrometer; the concentration required to suppress ROS by 50% was 10 micrometer for Cys and 50 micrometer for Met. Using metabolite kinetics with the assumption that Cys and Met are the metabolites of GSH, expected concentrations of Cys and Met of above 20 and 50 micrometer were estimated when GSH was administered at 50 mg/kg body weights every 205.4 min for Cys and 427.4 min for Met.
Keyword
MeSH Terms
Figure
Reference
-
1. Proudfoot AT, Prescott LF, Jarvie DR. Haemodialysis for paraquat poisoning. Hum Toxicol. 1987. 6:69–74.
Article2. Okonek S, Hofmann A, Henningsen B. Efficacy of gut lavage, hemodialysis, and hemoperfusion in the therapy of paraquat or diquat intoxication. Arch Toxicol. 1976. 36:43–51.
Article3. Meredith TJ, Vale JA. Treatment of paraquat poisoning in man: methods to prevent absorption. Hum Toxicol. 1987. 6:49–55.
Article4. Okonek S. Hemoperfusion in toxicology. Basic considerations of its effectiveness. Clin Toxicol. 1981. 18:1185–1198.
Article5. Lock EA, Smith LL, Rose MS. Inhibition of paraquat accumulation in rat lung slices by a component of rat plasma and a variety of drugs and endogenous amines. Biochem Pharmacol. 1976. 25:1769–1772.
Article6. Ross JH, Krieger RI. Structure-activity correlations of amines inhibiting active uptake of paraquat (methyl viologen) into rat lung slices. Toxicol Appl Pharmacol. 1981. 59:238–249.
Article7. Kitazawa Y, Matsubara M, Takeyama N, Tanaka T. The role of xanthine oxidase in paraquat intoxication. Arch Biochem Biophys. 1991. 288:220–224.
Article8. Webb DB, Williams MV, Davies BH, James KW. Resolution after radiotherapy of severe pulmonary damage due to paraquat poisoning. Br Med J (Clin Res Ed). 1984. 288:1259–1260.
Article9. Lin JL, Leu ML, Liu YC, Chen GH. A prospective clinical trial of pulse therapy with glucocorticoid and cyclophosphamide in moderate to severe paraquat-poisoned patients. Am J Respir Crit Care Med. 1999. 159:357–360.
Article10. Bus JS, Cagen SZ, Olgaard M, Gibson JE. A mechanism of paraquat toxicity in mice and rats. Toxicol Appl Pharmacol. 1976. 35:501–513.
Article11. Hagen TM, Brown LA, Jones DP. Protection against paraquat-induced injury by exogenous GSH in pulmonary alveolar type II cells. Biochem Pharmacol. 1986. 35:4537–4542.
Article12. Brown LA, Bai C, Jones DP. Glutathione protection in alveolar type II cells from fetal and neonatal rabbits. Am J Physiol. 1992. 262:L305–L312.
Article13. Meister A, Anderson ME. Glutathione. Annu Rev Biochem. 1983. 52:711–760.
Article14. Jurima-Romet M, Barber RF, Demeester J, Shek PN. Distribution studies of liposome-encapsulated glutathione administered to the lung. Int J Pharm. 1990. 63:227–235.15. Smith LJ, Anderson J, Shamsuddin M. Glutathione localization and distribution after intratracheal instillation. Implications for treatment. Am Rev Respir Dis. 1992. 145:153–159.
Article16. Reed DJ, Ellis WW, Meck RA. The inhibition of gamma-glutamyl transpeptidase and glutathione mtabolism of isolated rat kidney cells by L-(alpha S, 5S)-alpha-amino-3-chloro-4, 5-dihydro-5-isoxazoleacetic acid (AT-125; NSC-163501). Biochem Biophys Res Commun. 1980. 94:1273–1277.17. Rajpert-De Meyts E, Shi M, Chang M, Robison TW, Groffen J, Heisterkamp N, Forman HJ. Transfection with gamma-glutamyl transpeptidase enhances recovery from glutathione depletion using extracellular glutathione. Toxicol Appl Pharmacol. 1992. 114:56–62.18. Mudd SH, Levy JL. Stanbury J.B, editor. Disorders of transsulphuration. Metabolic bases of inherited disease. 1982. 5th ed. New York: McGraw-Hill;522–559.19. Stipanuk MH, Benevenga NJ. Effect of cystine on the metabolism of methionine in rats. J Nutr. 1977. 107:1455–1467.
Article20. Storch KJ, Wagner DA, Burke JF, Young VR. [1-13C; methyl-2H3] methionine kinetics in humans: methionine conservation and cystine sparing. Am J Physiol. 1990. 258:E790–E798.21. Young VR, Wagner DA, Burini R, Storch KJ. Methionine kinetics and balance at the 1985 FAO/WHO/UNU intake requirement in adult men studied with L-[2H3-methyl-1-13C]methionine as a tracer. Am J Clin Nutr. 1991. 54:377–385.
Article22. Aebi S, Assereto R, Lauterburg BH. High-dose intravenous glutathione in man. Pharmacokinetics and effects on cyst(e)ine in plasma and urine. Eur J Clin Invest. 1991. 21:103–110.
Article23. Koo HY, Shin I, Lee ZW, Lee SH, Kim SH, Lee CH, Kang HS, Ha KS. Roles of RhoA and phospholipase A2 in the elevation of intracellular H2O2 by transforming growth factor-β in Swiss 3T3 fibroblasts. Cell Signal. 1999. 11:677–683.24. Neuschwander-Tetri BA, Roll FJ. Glutathione measurement by high-performance liquid chromatography separation and fluorometric detection of the glutathione-orthophthalaldehyde adduct. Anal Biochem. 1989. 179:236–241.
Article25. Cereser C, Guichard J, Drai J, Bannier E, Garcia I, Boget S, Parvaz P, Revol A. Quantitation of reduced and total glutathione at the femtomole level by high-performance liquid chromatography with fluorescence detection: application to red blood cells and cultured fibroblasts. J Chromatogr B Biomed Sci Appl. 2001. 752:123–132.
Article26. Tarr GE. Shively J.E, editor. Methods of protein measurement. Microcharacterization. 1986. USA: Humana Press;155–194.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Circulating Total Glutathione in Normal Tension Glaucoma Patients: Comparison with Normal Control Subjects
- Protection of Hepatic Dysfunction during and after Hemorrhagic Shock with Intravenous Glutathione in Dogs
- Generation and characterization of a monoclonal antibody with high species-specificity to Schistosoma japonicum glutathione S-transferase
- Recent Updates on Acetaminophen Hepatotoxicity: The Role of Nrf2 in Hepatoprotection
- Changes of blood glutathione levels and RBC antioxidants activities in CRF patients