Korean J Ophthalmol.
2012 Apr;26(2):84-91. 10.3341/kjo.2012.26.2.84.
Circulating Total Glutathione in Normal Tension Glaucoma Patients: Comparison with Normal Control Subjects
- Affiliations
-
- 1Department of Ophthalmology, Yeouido St. Mary's Hospital, The Catholic University of Korea School of Medicine, Seoul, Korea. jimoon@catholic.ac.kr
- KMID: 1364860
- DOI: http://doi.org/10.3341/kjo.2012.26.2.84
Abstract
- PURPOSE
Oxidative stress plays a critical role in the pathogenesis of glaucoma. Glutathione is a major antioxidant molecule present in intracellular or extracellular space. Herein, we aimed to examine circulating glutathione level in normal tension glaucoma (NTG), which comprises the largest proportion of glaucoma disease in the Korean population.
METHODS
Nineteen NTG patients (NTG group) and 30 age- and gender-matched normal control subjects (control group) were included. Antecubital venous puncture was performed between 8 and 10 o'clock in the morning to obtain a 4 mL venous blood sample. Total glutathione level was measured by the spectrophotometric method at 412 nm. Correlation of total glutathione level with mean deviation and pattern standard deviation from the Humphrey visual field test was analyzed in the NTG group.
RESULTS
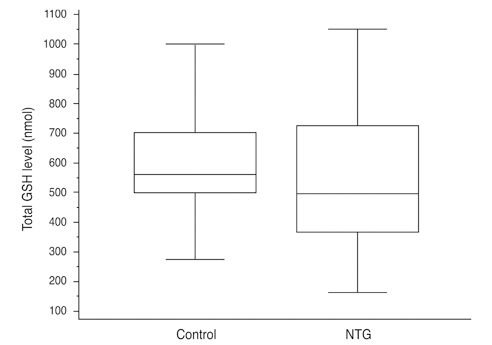
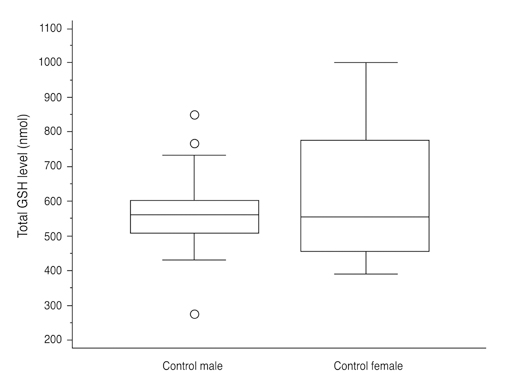
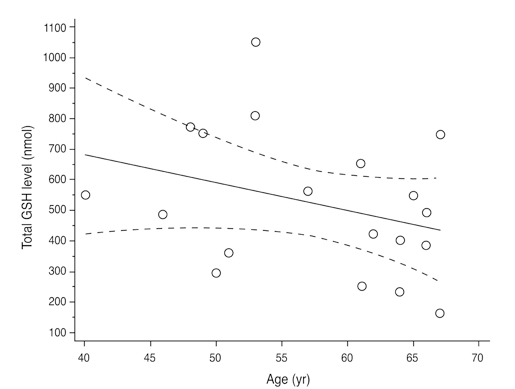
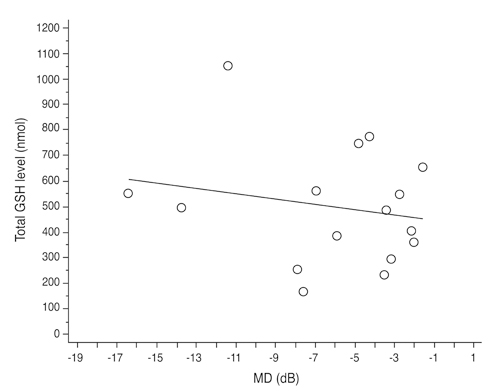
Total glutathione level in circulating blood was 524.02 +/- 231.09 nmol and 586.06 +/- 156.08 nmol in the NTG group and the control group, respectively. The difference between these values was not statistically significant (p = 0.121, F = 2.212). Age had no significant effect on circulating total glutathione level in either the NTG group (p = 0.171, r = -0.328) or the control group (p = 0.380, r = -0.166). In the NTG group, circulating total glutathione level had no significant relationship with mean deviation (p = 0.226, F = 1.636) and pattern standard deviation (p = 0.200, F = 1.766) after correcting for age and gender.
CONCLUSIONS
In NTG patients, circulating total glutathione levels were not different compared to those of normal subjects.
Keyword
MeSH Terms
Figure
Reference
-
1. Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006. 90:262–267.2. Liesegang TJ. Glaucoma: changing concepts and future directions. Mayo Clin Proc. 1996. 71:689–694.3. Quigley HA, Green WR. The histology of human glaucoma cupping and optic nerve damage: clinicopathologic correlation in 21 eyes. Ophthalmology. 1979. 86:1803–1830.4. Krakau CE. Intraocular pressure elevation-cause or effect in chronic glaucoma? Ophthalmologica. 1981. 182:141–147.5. Shen F, Chen B, Danias J, et al. Glutamate-induced glutamine synthetase expression in retinal Muller cells after short-term ocular hypertension in the rat. Invest Ophthalmol Vis Sci. 2004. 45:3107–3112.6. Chung HS, Harris A, Evans DW, et al. Vascular aspects in the pathophysiology of glaucomatous optic neuropathy. Surv Ophthalmol. 1999. 43:Suppl 1. S43–S50.7. Flammer J, Orgul S, Costa VP, et al. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res. 2002. 21:359–393.8. Butt Z, O'Brien C, McKillop G, et al. Color Doppler imaging in untreated high- and normal-pressure open-angle glaucoma. Invest Ophthalmol Vis Sci. 1997. 38:690–696.9. Findl O, Rainer G, Dallinger S, et al. Assessment of optic disk blood flow in patients with open-angle glaucoma. Am J Ophthalmol. 2000. 130:589–596.10. Flammer J, Haefliger IO, Orgul S, Resink T. Vascular dysregulation: a principal risk factor for glaucomatous damage? J Glaucoma. 1999. 8:212–219.11. Flammer J, Mozaffarieh M. What is the present pathogenetic concept of glaucomatous optic neuropathy? Surv Ophthalmol. 2007. 52:Suppl 2. S162–S173.12. Moreno MC, Campanelli J, Sande P, et al. Retinal oxidative stress induced by high intraocular pressure. Free Radic Biol Med. 2004. 37:803–812.13. Galassi F, Renieri G, Sodi A, et al. Nitric oxide proxies and ocular perfusion pressure in primary open angle glaucoma. Br J Ophthalmol. 2004. 88:757–760.14. Alvarado J, Murphy C, Polansky J, Juster R. Age-related changes in trabecular meshwork cellularity. Invest Ophthalmol Vis Sci. 1981. 21:714–727.15. Alvarado J, Murphy C, Juster R. Trabecular meshwork cellularity in primary open-angle glaucoma and nonglaucomatous normals. Ophthalmology. 1984. 91:564–579.16. Zhou L, Li Y, Yue BY. Oxidative stress affects cytoskeletal structure and cell-matrix interactions in cells from an ocular tissue: the trabecular meshwork. J Cell Physiol. 1999. 180:182–189.17. Sacca SC, Pascotto A, Camicione P, et al. Oxidative DNA damage in the human trabecular meshwork: clinical correlation in patients with primary open-angle glaucoma. Arch Ophthalmol. 2005. 123:458–463.18. Wang N, Chintala SK, Fini ME, Schuman JS. Activation of a tissue-specific stress response in the aqueous outflow pathway of the eye defines the glaucoma disease phenotype. Nat Med. 2001. 7:304–309.19. Sacca SC, Izzotti A, Rossi P, Traverso C. Glaucomatous outflow pathway and oxidative stress. Exp Eye Res. 2007. 84:389–399.20. Izzotti A, Bagnis A, Sacca SC. The role of oxidative stress in glaucoma. Mutat Res. 2006. 612:105–114.21. Ko ML, Hu DN, Ritch R, Sharma SC. The combined effect of brain-derived neurotrophic factor and a free radical scavenger in experimental glaucoma. Invest Ophthalmol Vis Sci. 2000. 41:2967–2971.22. Tanito M, Nishiyama A, Tanaka T, et al. Change of redox status and modulation by thiol replenishment in retinal photooxidative damage. Invest Ophthalmol Vis Sci. 2002. 43:2392–2400.23. Wangsa-Wirawan ND, Linsenmeier RA. Retinal oxygen: fundamental and clinical aspects. Arch Ophthalmol. 2003. 121:547–557.24. Siskova A, Wilhelm J. The effects of hyperoxia, hypoxia, and ischemia/reperfusion on the activity of cytochrome oxidase from the rat retina. Physiol Res. 2001. 50:267–273.25. Hirose F, Kiryu J, Miyamoto K, et al. In vivo evaluation of retinal injury after transient ischemia in hypertensive rats. Hypertension. 2004. 43:1098–1102.26. Hanashima C, Namiki H. Reduced viability of vascular endothelial cells by high concentration of ascorbic acid in vitreous humor. Cell Biol Int. 1999. 23:287–298.27. Brubaker RF, Bourne WM, Bachman LA, McLaren JW. Ascorbic acid content of human corneal epithelium. Invest Ophthalmol Vis Sci. 2000. 41:1681–1683.28. Dreyer R, Rose RC. Lacrimal gland uptake and metabolism of ascorbic acid. Proc Soc Exp Biol Med. 1993. 202:212–216.29. Ringvold A, Anderssen E, Kjonniksen I. Distribution of ascorbate in the anterior bovine eye. Invest Ophthalmol Vis Sci. 2000. 41:20–23.30. Giblin FJ, McCready JP, Kodama T, Reddy VN. A direct correlation between the levels of ascorbic acid and H2O2 in aqueous humor. Exp Eye Res. 1984. 38:87–93.31. Richer SP, Rose RC. Water soluble antioxidants in mammalian aqueous humor: interaction with UV B and hydrogen peroxide. Vision Res. 1998. 38:2881–2888.32. Kahn MG, Giblin FJ, Epstein DL. Glutathione in calf trabecular meshwork and its relation to aqueous humor outflow facility. Invest Ophthalmol Vis Sci. 1983. 24:1283–1287.33. Truscott RJ. Age-related nuclear cataract-oxidation is the key. Exp Eye Res. 2005. 80:709–725.34. Yildirim O, Ates NA, Tamer L, et al. Changes in antioxidant enzyme activity and malondialdehyde level in patients with age-related macular degeneration. Ophthalmologica. 2004. 218:202–206.35. Chen JZ, Kadlubar FF. A new clue to glaucoma pathogenesis. Am J Med. 2003. 114:697–698.36. Dringen R. Glutathione metabolism and oxidative stress in neurodegeneration. Eur J Biochem. 2000. 267:4903.37. Hall AG. Review: the role of glutathione in the regulation of apoptosis. Eur J Clin Invest. 1999. 29:238–245.38. Riley MV. Physiologic neutralization mechanisms and the response of the corneal endothelium to hydrogen peroxide. CLAO J. 1990. 16:1 Suppl. S16–S21.39. Costarides AP, Riley MV, Green K. Roles of catalase and the glutathione redox cycle in the regulation of anterior-chamber hydrogen peroxide. Ophthalmic Res. 1991. 23:284–294.40. Ferreira SM, Lerner SF, Brunzini R, et al. Oxidative stress markers in aqueous humor of glaucoma patients. Am J Ophthalmol. 2004. 137:62–69.41. Yang J, Tezel G, Patil RV, et al. Serum autoantibody against glutathione S-transferase in patients with glaucoma. Invest Ophthalmol Vis Sci. 2001. 42:1273–1276.42. Unal M, Guven M, Devranoglu K, et al. Glutathione S transferase M1 and T1 genetic polymorphisms are related to the risk of primary open-angle glaucoma: a study in a Turkish population. Br J Ophthalmol. 2007. 91:527–530.43. Gherghel D, Griffiths HR, Hilton EJ, et al. Systemic reduction in glutathione levels occurs in patients with primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2005. 46:877–883.44. Roh YJ, Moon C, Kim SY, et al. Glutathione depletion induces differential apoptosis in cells of mouse retina, in vivo. Neurosci Lett. 2007. 417:266–270.45. Park JW, Moon C, Yun S, et al. Differential expression of heat shock protein mRNAs under in vivo glutathione depletion in the mouse retina. Neurosci Lett. 2007. 413:260–264.46. Choe YJ, Hong YJ. The prevalence of glaucoma in Korean careermen. J Korean Ophthalmol Soc. 1993. 34:153–158.47. Iwase A, Suzuki Y, Araie M, et al. The prevalence of primary open-angle glaucoma in Japanese: the Tajimi Study. Ophthalmology. 2004. 111:1641–1648.48. Kim CS, Seong GJ, Lee NH, et al. Prevalence of primary open-angle glaucoma in central South Korea the Namil study. Ophthalmology. 2011. 118:1024–1030.49. Collaborative Normal-Tension Glaucoma Study Group. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol. 1998. 126:487–497.50. Collaborative Normal-Tension Glaucoma Study Group. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am J Ophthalmol. 1998. 126:498–505.51. Allingham RR, Shields MB, editors. Neuroprotection and other investigational drugs. Shields' textbook of glaucoma. 2005. Philadelphia: Lippincott Williams & Wilkins;512–515.52. Brubaker RF. Delayed functional loss in glaucoma: LII Edward Jackson Memorial Lecture. Am J Ophthalmol. 1996. 121:473–483.53. Akerboom TP, Sies H. Assay of glutathione, glutathione disulfide, and glutathione mixed disulfides in biological samples. Methods Enzymol. 1981. 77:373–382.54. Nguyen KP, Weiss H, Karageuzian LN, et al. Glutathione reductase of calf trabecular meshwork. Invest Ophthalmol Vis Sci. 1985. 26:887–890.55. Levin LA, Clark JA, Johns LK. Effect of lipid peroxidation inhibition on retinal ganglion cell death. Invest Ophthalmol Vis Sci. 1996. 37:2744–2749.56. Izzotti A, Sacca SC, Cartiglia C, De Flora S. Oxidative deoxyribonucleic acid damage in the eyes of glaucoma patients. Am J Med. 2003. 114:638–646.57. Schutte M, Werner P. Redistribution of glutathione in the ischemic rat retina. Neurosci Lett. 1998. 246:53–56.58. Carter-Dawson L, Shen FF, Harwerth RS, et al. Glutathione content is altered in Muller cells of monkey eyes with experimental glaucoma. Neurosci Lett. 2004. 364:7–10.59. Kim SH, Park HM, Seo JH, Hur M. The change of blood reduced glutathione according to postmenopausal HRT: GSH as a marker of antioxidant effect of the sex steroids. J Korean Soc Menopause. 1997. 3:116–125.60. Park DK, Jeong SK, Chung MG, et al. Glutathione levels in Helicobacter pylori-infected gastric mucosa. Korean J Gastroenterol. 2003. 42:267–273.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of the Central Retinal Vessel Diameter Between Glaucomatous and Normal Eye
- Analysis of Systemic Risk Factorsin Normal Tension Glaucoma
- Comparison of Dietary Patterns Between Glaucoma Patients and Normal Control Subjects
- Comparison of Serum Homocysteine, Vitamin B12, Vitamin B6 and Folate Levels in Different Glaucoma Types
- Significance of Peripapillary Atrophy in the Diagnosis of Open-angle Glaucoma