J Korean Med Sci.
2005 Feb;20(1):113-120. 10.3346/jkms.2005.20.1.113.
Effect of Ketamine on Apoptosis by Energy Deprivation in Astroglioma Cells using Flow Cytometry System
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. mhkim@smc.samsung.co.kr
- 2Department of Anesthesiology and Pain Medicine, Chungbuk National University, Cheongju, Korea.
- KMID: 1781737
- DOI: http://doi.org/10.3346/jkms.2005.20.1.113
Abstract
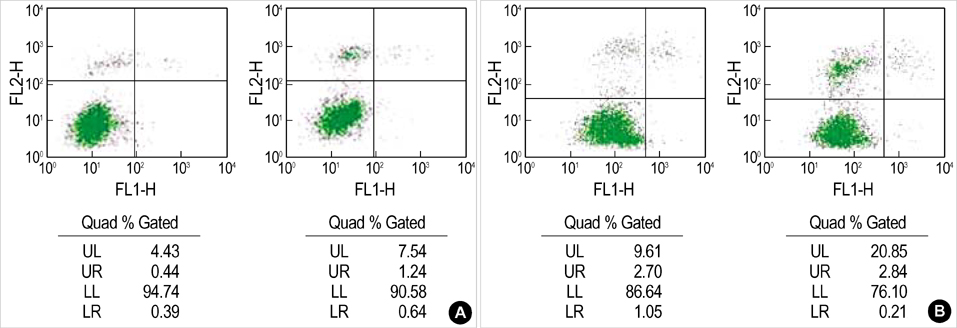
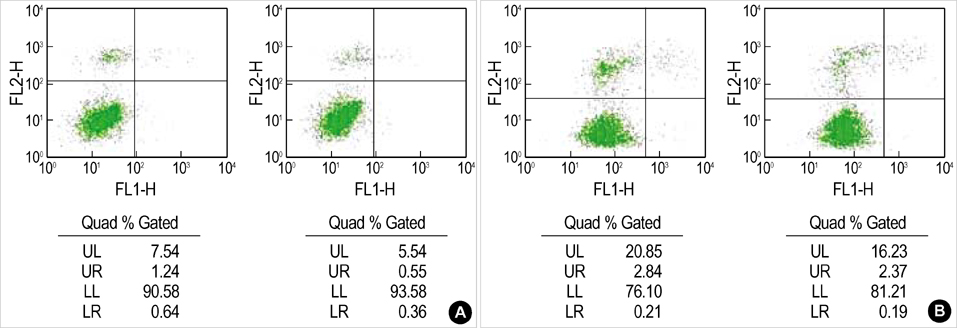
- Apoptosis is a programmed, physiologic mode of cell death that plays an important role in tissue homeostasis. As for the central nervous system, ischemic insults can induce pathophysiologic cascade of apoptosis in neurophils. Impairment of astroctye functions during brain ischemia can critically influence neuron survival by neuronglia interactions. We aimed to elucidate the protective effect of ketamine on apoptosis by energy deprivation in astrocytes. Ischemic insults was induced with iodoacetate/ carbonylcyanide mchlorophenylhydrazone (IAA/CCCP) 1.5 mM/ 20 micrometer or 150 micrometer/2 micrometer for 1 hr in the HTB-15 and CRL-1690 astrocytoma cells. Then these cells were reperfused with normal media or ketamine (0.1 mM) containing media for 1 hr or 24 hr. FITC-annexin-V staining and propidium iodide binding were determined by using flow cytometry. Cell size and granularity were measured by forward and side light scattering properties of flow cytometry system, respectively. An addition of keta-mine during reperfusion increased the proportion of viable cells. Ketamine alleviated cell shrinkage and increased granularity during the early period, and ameliorated cell swelling during the late reperfusion period. Ketamine may have a valuable effect on amelioration of early and late apoptosis in the astrocytoma cells, even though the exact mechanism remains to be verified.
Keyword
MeSH Terms
-
Anesthetics, Dissociative/*pharmacology
Annexin A5/pharmacology
Apoptosis
Astrocytes/metabolism
Astrocytoma/*drug therapy/pathology
Brain/pathology
Carbonyl Cyanide m-Chlorophenyl Hydrazone/pharmacology
Cell Line, Tumor
Cell Size
Cell Survival
Central Nervous System/drug effects/pathology
Enzyme Inhibitors/pharmacology
Flow Cytometry/*methods
Humans
Indicators and Reagents/pharmacology
Iodoacetates/pharmacology
Ischemia/pathology
Ketamine/metabolism/*pharmacology
Light
Neurons/metabolism/pathology
Neutrophils/metabolism
Perfusion
Propidium/pharmacology
Scattering, Radiation
Time Factors
Uncoupling Agents/pharmacology
Figure
Reference
-
1. Reno F, Burattini S, Rossi S, Luchetti F, Columbaro M, Santi S, Papa S, Falcieri E. Phospholipid rearrangement of apoptotic membrane does not depend on nuclear activity. Histochem Cell Biol. 1998. 110:467–476.2. Walton M, Sirimanne E, Reutelingsperger C, Williams C, Gluckman P, Dragunow M. Annexin V labels apoptotic neurons following hypoxia-ischemia. Neuroreport. 1997. 8:3871–3875.
Article3. Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, van Schie RC, LaFace DM, Green DR. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995. 182:1545–1556.
Article4. Rimon G, Bazenet CE, Philpott KL, Rubin LL. Increased surface phosphatidylserine is an early marker of neuronal apoptosis. J Neurosci Res. 1997. 48:563–570.
Article5. van Engeland M, Ramaekers FC, Schutte B, Reutelingsperger CP. A novel assay to measure loss of plasma membrane asymmetry during apoptosis of adherent cells in culture. Cytometry. 1996. 24:131–139.
Article6. Bose S, Tuunainen I, Parry M, Medina OP, Mancini G, Kinnunen PK. Binding of cationic liposomes to apoptotic cells. Anal Biochem. 2004. 331:385–394.
Article7. Telford WG, King LE, Fraker PJ. Rapid quantitation of apoptosis in pure and heterogeneous cell populations using flow cytometry. J Immunol Methods. 1994. 172:1–16.
Article8. Shapira Y, Lam AM, Eng CC, Laohapraist V, Michel M. Therapeutic time window and response of the beneficial effects of ketamine in experimental head injury. Stroke. 1994. 25:1637–1643.9. Rothman SM, Thurston JH, Hauhart RE, Clark GD, Solomon JS. Ketamine protects hippocampal neurons from anoxia in vitro. Neuroscience. 1987. 21:673–678.
Article10. Pophprecki R, Becker GL, Reilly PJ, Landers DF. Ketamine, MK-801 or calmidazolium protects rat hippocampal energy status during in vitro ischemia. Ann NY Acad Sci. 1991. 625:818–820.
Article11. Lin SZ, Chiou AL, Wang Y. Ketamine antagonizes nitric oxide release from cerebral cortex after middle cerebral artery ligation in rats. Stroke. 1996. 27:747–752.
Article12. Hoffman WE, Peligrino D, Werner C, Kochs E, Albrecht RF, Schulte am Esch J. Ketamine decreases plasma catecholamines and improves outcome from incomplete cerebral ischemia in rats. Anesthesiology. 1992. 76:755–762.
Article13. Chen Y, Swanson RA. Astrocytes and brain injury. J Cereb Blood Flow Metab. 2003. 23:137–149.
Article14. Aschner M, Sonnewald U, Tan KH. Astrocyte modulation of neurotoxic injury. Brain Pathol. 2002. 12:475–481.
Article15. Tamatani M, Ogawa S, Niitsu Y, Tohyama M. Involvement of Bcl-2 family and caspase-3 like protease in NO-mediated neuronal apoptosis. J Neurochem. 1998. 71:1588–1596.16. Ohgoh M, Kimura M, Ogura H, Katayama K, Nishizawa Y. Apoptotic cell death of cultured cerebral cortical neurons induced by withdrawal of astroglial trophic support. Exp Neurol. 1998. 149:51–63.
Article17. Wang XF, Cynader MS. Effects of astrocytes on neuronal attachment and survival shown in a serum-free co-culture system. Brain Res Protoc. 1999. 4:209–216.
Article18. Araque A, Carmignoto G, Haydon PG. Dynamic signaling between astrocytes and neurons. Annu Rev Physiol. 2001. 63:795–813.
Article19. Healy E, Dempsey M, Lally C, Ryan MP. Apoptosis and necrosis: mechanisms of cell death induced by cyclosporine A in a renal proximal tubular cell line. Kidney Int. 1998. 54:1955–1966.
Article20. Yu AC, Wong HK, Yung HW, Lau LT. Ischemia-induced apoptosis in primary cultures of astrocytes. Glia. 2001. 35:121–130.
Article21. Gummadi SN, Hrafnsdottir S, Walent J, Watkins WE, Menon AK. Reconstitution and assay of biogenic membrane-derived phospholipid flippase activity in proteoliposomes. Methods Mol Biol. 2003. 228:271–279.
Article22. Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in-situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992. 119:493–501.23. Zhang G, Gurtu V, Kain SR, Yan G. Early detection of apoptosis using a fluorescent conjugate of annexin-V. Biotechniques. 1997. 23:525–531.24. Chan A, Reiter R, Wiese S, Fertig G, Gold R. Plasma membrane phospholipid asymmetry precedes DNA fragmentation in different apoptotic cell models. Histochem Cell Biol. 1998. 110:553–558.
Article25. Castedo M, Hirsch T, Susin SA, Zamzami N, Marchetti P, Macho A, Kroemer G. Sequential acquisition of mitochondrial and plasma membrane alterations during early lymphocyte apoptosis. J Immunol. 1996. 157:512–521.26. van Engeland M, Nieland LJ, Ramaekers FC, Schutte B, Reutelingsperger CP. Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry. 1998. 31:1–9.
Article27. Smis NR, Anderson MF. Mitochondrial contributions to tissue damage in stroke. Neurochem Int. 2002. 40:511–526.28. Wolvetang EJ, Johnson KL, Krauer K, Ralph SJ, Linnane AW. Mitochondrial respiratory chain inhibitors induce apoptosis. FEBS Lett. 1994. 339:40–44.
Article29. Zhou M, Ma T, Tseng MT. Effects of taurine and ketamine on bovine retinal membrane lipid peroxidation. Neuroscience. 1991. 45:461–465.
Article30. Bonfoco E, Kraine D, Ankarerona M, Nicotera P, Lipon SA. Apoptosis and necrosis: two distinct events induced, respectively, by mild and intense insults with N-methyl-D-aspartate or nitric oxide/superoxide in cortical cell cultures. Proc Natl Acad Sci USA. 1995. 92:7162–7166.
Article31. Leist M, Single B, Castoldi AF, Kuhnle S, Nicotera P. Intracellular adenosine triphosphate (ATP) concentration: A switch in the decision between apoptosis and necrosis. J Exp Med. 1997. 185:1481–1486.
Article32. Pfenninger E, Himmelseher S. Neuroprotection by ketamine at the cellular level. Anaesthesist. 1997. 46:S47–S54.33. Chen M, Simard JM. Cell swelling and a nonselective cation channel regulated by internal Ca2+ and ATP in native reactive astrocytes from adult rat brain. J Neurosci. 2001. 21:6512–6521.34. Kohrs R, Durieux ME. Ketamine: teaching an old drug new tricks. Anesth Analg. 1998. 87:1186–1193.35. Krebs C, Fernandes HB, Sheldon C, Raymond LA, Baimbridge KG. Functional NMDA receptor subtype 2B is expressed in astrocytes after ischemia in vivo and anoxia in vitro. J Neurosci. 2003. 23:3364–3372.36. Kim MH, Hahm TS, Ahn HJ. Regulation of astroglial volume by ketamine in glutamate induced cellular volume changes. Korean J Anesthesiol. 1997. 33:1005–1011.
Article37. Chan PH, Chu L. Ketamine protects cultured astrocytes from glutamate-induced swelling. Brain Res. 1989. 487:380–383.
Article38. Brau ME, Sander F, Vogel W, Hempelmann G. Blocking mechanisms of ketamine and its enantiomers in enzymatically demyelinated peripheral nerve as revealed by single-channel experiments. Anesthesiology. 1997. 86:394–404.
Article39. Silver IA, Erecinska M. Intracellular and extracellular changes of Ca2+ in hypoxia and ischemia in rat brain in vivo. J Gen Physiol. 1990. 95:837–866.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Analysis of T Cells Using Flow Cytometry
- Effect of Creatine on the Survival of RGC-5 Cells under Serum Deprivation
- A Combination of PG490 and Lipopolysaccharide Induce Apoptosis through Activation of Casapase-3 and Down- regulation of cIAP1 and XIAP in Human Astroglioma Cell
- Caspases Activation in Ultraviolet B-induced Apoptosis of G361 Human Melanoma Cell Line
- Regulation of Astroglial Volume by Ketamine in Glutamate Induced Cellular Volume Changes